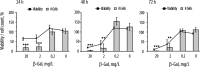Enzyme Prodrug Therapy Achieves Site-Specific, Personalized Physiological Responses to the Locally Produced Nitric Oxide - PubMed (original) (raw)
Enzyme Prodrug Therapy Achieves Site-Specific, Personalized Physiological Responses to the Locally Produced Nitric Oxide
Anna K Winther et al. ACS Appl Mater Interfaces. 2018.
Abstract
Nitric oxide (NO) is a highly potent but short-lived endogenous radical with a wide spectrum of physiological activities. In this work, we developed an enzymatic approach to the site-specific synthesis of NO mediated by biocatalytic surface coatings. Multilayered polyelectrolyte films were optimized as host compartments for the immobilized β-galactosidase (β-Gal) enzyme through a screen of eight polycations and eight polyanions. The lead composition was used to achieve localized production of NO through the addition of β-Gal-NONOate, a prodrug that releases NO following enzymatic bioconversion. The resulting coatings afforded physiologically relevant flux of NO matching that of the healthy human endothelium. The antiproliferative effect due to the synthesized NO in cell culture was site-specific: within a multiwell dish with freely shared media and nutrients, a 10-fold inhibition of cell growth was achieved on top of the biocatalytic coatings compared to the immediately adjacent enzyme-free microwells. The physiological effect of NO produced via the enzyme prodrug therapy was validated ex vivo in isolated arteries through the measurement of vasodilation. Biocatalytic coatings were deposited on wires produced using alloys used in clinical practice and successfully mediated a NONOate concentration-dependent vasodilation in the small arteries of rats. The results of this study present an exciting opportunity to manufacture implantable biomaterials with physiological responses controlled to the desired level for personalized treatment.
Keywords: biocatalytic coating; enzyme-prodrug therapy; galactosidase; nitric oxide; polyelectrolyte multilayers; stent; vasodilatation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Figure 1
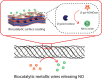
Enzymatic synthesis of NO is engineered in this work into multilayered polyelectrolyte coatings. When used as substrates for cell culture, these biocatalytic coatings provide localized synthesis of NO for localized delivery to the adhering cells.
Figure 2
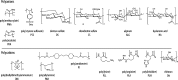
Chemical structure and abbreviations of polyanions and polycations used in this study (DNA and PRT not shown).
Figure 3
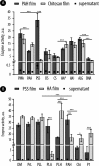
Results of quantification of the catalytic output for the multilayered coatings composed of different polyanion/polycation combinations and equipped with β-Gal: (A) multilayered coatings with PAH or Chi as polycations and a variation of polyanions; (B) multilayers with PSS or HA as polyanions and a variation of polycations. HA with high and low molar mass is denoted as HAH and HAL, respectively. Enzymatic catalysis was evaluated using a fluorogenic enzyme substrate, resorufin β-
d
-galactopyranoside.
Figure 4
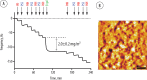
(A) QCM monitoring of the assembly of multilayered surface coatings based on PSS and PAH (PEI priming layer) and immobilization of β-Gal. Quantification of protein coverage is based on three independent experiments. For experimental details, see the Materials and Methods. (B) AFM image of the PSS/PAH coating with immobilized β-Gal. Scale bars: 300 nm (black, XY dimension) and 0–6 nm (_Z-_direction).
Figure 5
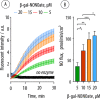
(A) Kinetic curves illustrating the evolution of fluorescence resulting from the biocatalytic production of NO by the multilayered surface coatings at varied concentrations of β-Gal–NONOate and subsequent conversion of DAF-FM into its fluorescent product. (B) Flux of NO afforded by the biocatalytic surface coatings at varied concentrations of β-Gal–NONOate (calculated from the linear part of the data curves in panel (A)).
Figure 6
(A) Kinetic curves illustrating the evolution of fluorescence resulting from the reaction of DAF-FM with NO produced by the biocatalytic surface coatings. Coatings were assembled using enzyme feed solutions, with the protein content from 0.2 to 20 mg/L; 100 μM β-Gal–NONOate. (B) NO flux sustained by the biocatalytic coatings (calculated from the linear part of the curves in panel (A)). (C) NO flux sustained by the biocatalytic surface coatings assembled using 20 mg/L enzyme feed solution in the presence of 100 μM β-Gal–NONOate as measured at the time points from 1 to 4 days.
Figure 7
Cell number and viability for myoblasts cultured on the PSS/PAH multilayered polyelectrolyte films over 24, 48, and 72 h. Multilayered films were equipped with the β-Gal enzyme with the feed protein content of 0, 0.2, 2, and 20 mg/L. Cell culture was performed in the presence of 100 μM NONOate. Results are presented as means ± SD for at least three independent experiments.
Figure 8
Fluorescence microscopy images of myoblast cells proliferating on the multilayered polyelectrolyte coatings in the presence of 100 μM NONOate over 72 h of cell culture either with or without β-Gal incorporated into the polymer film. Scale bar: 50 μm.
Figure 9
(A) Schematic representation of coculture μ-slides indicating the multilayered-coated wells. (B) Fluorescence microscopy imaging of myoblast cells. Selected wells were coated with biocatalytic multilayers with 20 mg/L β-Gal for local delivery of NO. Cells were incubated for 48 h in the presence of 100 μM NONOate, replenished at 24 h. Scale bar: 100 μm. (C) Averaged cell count of coated vs noncoated wells. Results are presented as mean ± SD for at least three independent experiments. ***P < 0.001.
Figure 10
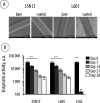
(A) Scanning electron microscopy images of the 35N LT and L605 wires in pristine form (bare) and after the assembly of a biocatalytic coating; scale bars: 100 μm. (B) Enzymatic activity of the multilayered polyelectrolyte coatings containing β-Gal and assembled on the corrosion-resistant alloys (35N LT, L605, 316L) during incubation in PBS at 37 °C over 28 days. Statistical evaluation was performed to compare the enzymatic activity of the coated wires with the background fluorescence of a metal without the enzyme, calculated via a two-way ANOVA, followed by Tukey’s multiple comparison test.
Figure 11
Ex vivo wire myograph quantification of the contraction force exerted ex vivo by the rat mesenteric arteries (A,B) and calculated degree of vasorelaxation (C,D) in the presence of NONOate (0.5 nM to 15 μM) and the wires based on 35N LT and L605 alloys coated with the biocatalytic multilayered polyelectrolyte coatings (denoted as wire + Enz + NONOate). Control experiments include administering the NONOate in the absence of wires (denoted as NONOate), using the wires and multilayered coatings with no incorporated enzyme (denoted wire + NONOate), and using the samples identical to the experimental group and also containing specific inhibitors of the NO-mediated signaling pathways (denoted as wire + Enz + NONOate + ODQ/IbTX). Data are presented as mean ± SEM, n = 5 or greater. Statistics is shown for comparing the effects mediated by the biocatalytic coatings with those mediated by the NONOate (¤), the coatings with no enzyme (#), and the biocatalytic coatings in the presence of inhibitors (*) and calculated via a two-way ANOVA followed by Tukey’s multiple comparison test.
Similar articles
- Biocatalytic polymer thin films: optimization of the multilayered architecture towards in situ synthesis of anti-proliferative drugs.
Andreasen SØ, Fejerskov B, Zelikin AN. Andreasen SØ, et al. Nanoscale. 2014 Apr 21;6(8):4131-40. doi: 10.1039/c3nr05999e. Nanoscale. 2014. PMID: 24604061 - Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug.
Wang Z, Lu Y, Qin K, Wu Y, Tian Y, Wang J, Zhang J, Hou J, Cui Y, Wang K, Shen J, Xu Q, Kong D, Zhao Q. Wang Z, et al. J Control Release. 2015 Jul 28;210:179-88. doi: 10.1016/j.jconrel.2015.05.283. Epub 2015 May 22. J Control Release. 2015. PMID: 26004323 - Gene transfer of endothelial nitric oxide isoform decreases rat hindlimb vascular resistance in vivo.
Gaballa MA, Goldman S. Gaballa MA, et al. Hum Gene Ther. 2000 Aug 10;11(12):1637-46. doi: 10.1089/10430340050111296. Hum Gene Ther. 2000. PMID: 10954898 - Recent developments in nitric oxide-releasing biomaterials for biomedical applications.
Yu H, Cui LX, Huang N, Yang ZL. Yu H, et al. Med Gas Res. 2019 Oct-Dec;9(4):184-191. doi: 10.4103/2045-9912.273956. Med Gas Res. 2019. PMID: 31898603 Free PMC article. Review. - Enzyme Mimics for the Catalytic Generation of Nitric Oxide from Endogenous Prodrugs.
Yang T, Zelikin AN, Chandrawati R. Yang T, et al. Small. 2020 Jul;16(27):e1907635. doi: 10.1002/smll.201907635. Epub 2020 May 5. Small. 2020. PMID: 32372556 Review.
Cited by
- Structural heterogeneity in polymeric nitric oxide donor nanoblended coatings for controlled release behaviors.
Jeong H, Park K, Yoo JC, Hong J. Jeong H, et al. RSC Adv. 2018 Nov 19;8(68):38792-38800. doi: 10.1039/c8ra07707j. eCollection 2018 Nov 16. RSC Adv. 2018. PMID: 35558288 Free PMC article. - Combatting implant-associated biofilms through localized drug synthesis.
Walther R, Nielsen SM, Christiansen R, Meyer RL, Zelikin AN. Walther R, et al. J Control Release. 2018 Oct 10;287:94-102. doi: 10.1016/j.jconrel.2018.08.025. Epub 2018 Aug 20. J Control Release. 2018. PMID: 30138714 Free PMC article. - Nitric Oxide to Fight Viral Infections.
Lisi F, Zelikin AN, Chandrawati R. Lisi F, et al. Adv Sci (Weinh). 2021 Feb 9;8(7):2003895. doi: 10.1002/advs.202003895. eCollection 2021 Apr. Adv Sci (Weinh). 2021. PMID: 33850691 Free PMC article. Review. - Recent Developments in Layer-by-Layer Assembly for Drug Delivery and Tissue Engineering Applications.
Borges J, Zeng J, Liu XQ, Chang H, Monge C, Garot C, Ren KF, Machillot P, Vrana NE, Lavalle P, Akagi T, Matsusaki M, Ji J, Akashi M, Mano JF, Gribova V, Picart C. Borges J, et al. Adv Healthc Mater. 2024 Mar;13(8):e2302713. doi: 10.1002/adhm.202302713. Epub 2024 Jan 7. Adv Healthc Mater. 2024. PMID: 38116714 Free PMC article. Review. - Nitric Oxide-Producing Cardiovascular Stent Coatings for Prevention of Thrombosis and Restenosis.
Rao J, Pan Bei H, Yang Y, Liu Y, Lin H, Zhao X. Rao J, et al. Front Bioeng Biotechnol. 2020 Jun 24;8:578. doi: 10.3389/fbioe.2020.00578. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32671029 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources