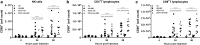Matrix-M™ adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice - PubMed (original) (raw)
Matrix-M™ adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice
Sofia E Magnusson et al. Immunol Res. 2018 Apr.
Abstract
Influenza viruses continuously circulate in the human population and escape recognition by virus neutralizing antibodies induced by prior infection or vaccination through accumulation of mutations in the surface proteins hemagglutinin (HA) and neuraminidase (NA). Various strategies to develop a vaccine that provides broad protection against different influenza A viruses are under investigation, including use of recombinant (r) viral vectors and adjuvants. The replication-deficient modified vaccinia virus Ankara (MVA) is a promising vaccine vector that efficiently induces B and T cell responses specific for the antigen of interest. It is assumed that live vaccine vectors do not require an adjuvant to be immunogenic as the vector already mediates recruitment and activation of immune cells. To address this topic, BALB/c mice were vaccinated with either protein- or rMVA-based HA influenza vaccines, formulated with or without the saponin-based Matrix-M™ adjuvant. Co-formulation with Matrix-M significantly increased HA vaccine immunogenicity, resulting in antigen-specific humoral and cellular immune responses comparable to those induced by unadjuvanted rMVA-HA. Of special interest, rMVA-HA immunogenicity was also enhanced by addition of Matrix-M, demonstrated by enhanced HA inhibition antibody titres and cellular immune responses. Matrix-M added to either protein- or rMVA-based HA vaccines mediated recruitment and activation of antigen-presenting cells and lymphocytes to the draining lymph node 24 and 48 h post-vaccination. Taken together, these results suggest that adjuvants can be used not only with protein-based vaccines but also in combination with rMVA to increase vaccine immunogenicity, which may be a step forward to generate new and more effective influenza vaccines.
Keywords: Immunogenicity; Influenza virus; MVA; Matrix-M™ adjuvant; Vaccine.
Conflict of interest statement
Conflict of interest
SEM, KLB, and LS are employees of Novavax and hold stock and/or stock options in the company. Other authors report no conflict of interest.
Figures
Fig. 1
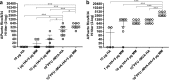
HA-specific antibody responses induced after vaccination with HA protein or rMVA-HA with or without Matrix-M adjuvant IgG1 (a) and IgG2a (b) HA-specific antibody responses 21 days after the primary vaccination. c–d IgG1 and IgG2a HA-specific antibody responses 14 days after the booster vaccination. IgG1 (a, c) or IgG2a (b, d) serum antibodies were detected by ELISA using purified HA protein and anti-IgG1 or anti-IgG2a HRP-conjugated antibodies. Data is shown as mean ± 95% confidence interval (CI). *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. MM = Matrix-M adjuvant
Fig. 2
Induction of HA-specific HI antibody responses after vaccination with rMVA-HA adjuvanted with Matrix-M HI serum antibody responses against influenza virus A/Puerto Rico/8/34 (H1N1) was measured 21 days after the primary (a) or 14 days after the booster (b) vaccination. Data is shown as mean ± 95% CI. *p < 0.05; **p < 0.01; ***p < 0.001. MM = Matrix-M adjuvant
Fig. 3
Enhanced HA-specific splenocyte responses by Matrix-M-adjuvanted vaccine spleens obtained 14 days after the booster vaccination were stimulated with purified HA protein and the number of IL-2 (a), IFN-γ (b), and IL-2/IFN-γ (c) producing splenocytes was determined in spot forming units (SFU)/106 cells by Fluorospot assay. Samples were tested in triplicate. The mean ± 95% CI of each group is indicated. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. MM = Matrix-M adjuvant
Fig. 4
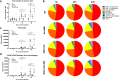
Matrix-M-adjuvanted influenza vaccines induce influx of immune cells in the dLN with maintained composition of cellular subsets except for an increased monocyte population. a The total number of cells per dLN. b Contribution (%) of the indicated cellular subsets in the dLN was measured by flow cytometry at 4, 24, or 48 h after i.m. vaccination. c–d Total cell count of CD11b+Ly6C+ monocytes (c) and CD169+F4/80+ medullary sinus macrophages (d) were determined by flow cytometry. Data are shown as mean of 10 mice per group. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. MM = Matrix-M adjuvant
Fig. 5
Increased activation of APCs in the dLN 24 and 48 h after vaccination with Matrix-M-adjuvanted influenza HA vaccines. a–b The number of CD69+ or CD86+ DCs, monocytes, and B lymphocytes recruited to the dLN was measured by flow cytometry 4, 24, and 48 h after i.m. injection of the respective vaccine. c The mean fluorescence intensity (MFI) of MHC class II of on DCs, monocytes, and B lymphocytes in the dLN was measured by flow cytometry 4, 24, and 48 h post-injection. Data are shown as mean of 10 mice per group. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. MM = Matrix-M adjuvant
Fig. 6
Increased number of activated NK- and T lymphocytes in the dLN at 24 and 48 h post-vaccination with Matrix-M-adjuvanted influenza HA vaccines The number of CD69+ NK cells (a), CD4+ (b), and CD8+ (c) T lymphocytes in the dLN was measured by flow cytometry 4, 24, and 48 h after i.m. injection of the respective vaccines. Data are shown as mean of 10 mice per group. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. MM = Matrix-M adjuvant
Similar articles
- Protein and modified vaccinia virus Ankara-based influenza virus nucleoprotein vaccines are differentially immunogenic in BALB/c mice.
Altenburg AF, Magnusson SE, Bosman F, Stertman L, de Vries RD, Rimmelzwaan GF. Altenburg AF, et al. Clin Exp Immunol. 2017 Oct;190(1):19-28. doi: 10.1111/cei.13004. Epub 2017 Jul 24. Clin Exp Immunol. 2017. PMID: 28665497 Free PMC article. - Immunization with recombinant modified vaccinia Ankara (rMVA) constructs encoding the HA or NP gene protects ponies from equine influenza virus challenge.
Breathnach CC, Clark HJ, Clark RC, Olsen CW, Townsend HG, Lunn DP. Breathnach CC, et al. Vaccine. 2006 Feb 20;24(8):1180-90. doi: 10.1016/j.vaccine.2005.08.091. Epub 2005 Sep 12. Vaccine. 2006. PMID: 16194586 - Immunogenicity and Protection Against Influenza H7N3 in Mice by Modified Vaccinia Virus Ankara Vectors Expressing Influenza Virus Hemagglutinin or Neuraminidase.
Meseda CA, Atukorale V, Soto J, Eichelberger MC, Gao J, Wang W, Weiss CD, Weir JP. Meseda CA, et al. Sci Rep. 2018 Mar 29;8(1):5364. doi: 10.1038/s41598-018-23712-9. Sci Rep. 2018. PMID: 29599502 Free PMC article. - Progress on adenovirus-vectored universal influenza vaccines.
Xiang K, Ying G, Yan Z, Shanshan Y, Lei Z, Hongjun L, Maosheng S. Xiang K, et al. Hum Vaccin Immunother. 2015;11(5):1209-22. doi: 10.1080/21645515.2015.1016674. Hum Vaccin Immunother. 2015. PMID: 25876176 Free PMC article. Review. - Modified vaccinia virus ankara (MVA) as production platform for vaccines against influenza and other viral respiratory diseases.
Altenburg AF, Kreijtz JH, de Vries RD, Song F, Fux R, Rimmelzwaan GF, Sutter G, Volz A. Altenburg AF, et al. Viruses. 2014 Jul 17;6(7):2735-61. doi: 10.3390/v6072735. Viruses. 2014. PMID: 25036462 Free PMC article. Review.
Cited by
- Development of semisynthetic saponin immunostimulants.
Bai D, Kim H, Wang P. Bai D, et al. Med Chem Res. 2024;33(8):1292-1306. doi: 10.1007/s00044-024-03227-x. Epub 2024 May 18. Med Chem Res. 2024. PMID: 39132259 Free PMC article. Review. - A Pfs48/45-based vaccine to block Plasmodium falciparum transmission: phase 1, open-label, clinical trial.
Alkema M, Smit MJ, Marin-Mogollon C, Totté K, Teelen K, van Gemert GJ, van de Vegte-Bolmer M, Mordmüller BG, Reimer JM, Lövgren-Bengtsson KL, Sauerwein RW, Bousema T, Plieskatt J, Theisen M, Jore MM, McCall MBB. Alkema M, et al. BMC Med. 2024 Apr 23;22(1):170. doi: 10.1186/s12916-024-03379-y. BMC Med. 2024. PMID: 38649867 Free PMC article. Clinical Trial. - Recent and advanced nano-technological strategies for COVID-19 vaccine development.
Nwagwu CS, Ugwu CN, Ogbonna JDN, Onugwu AL, Agbo CP, Echezona AC, Ezeibe EN, Uzondu S, Kenechukwu FC, Akpa PA, Momoh MA, Nnamani PO, Tarirai C, Ofokansi KC, Attama AA. Nwagwu CS, et al. Methods Microbiol. 2022;50:151-188. doi: 10.1016/bs.mim.2022.03.001. Epub 2022 Apr 18. Methods Microbiol. 2022. PMID: 38620863 Free PMC article. - Emulsion and liposome-based adjuvanted R21 vaccine formulations mediate protection against malaria through distinct immune mechanisms.
Reinke S, Pantazi E, Chappell GR, Sanchez-Martinez A, Guyon R, Fergusson JR, Salman AM, Aktar A, Mukhopadhyay E, Ventura RA, Auderset F, Dubois PM, Collin N, Hill AVS, Bezbradica JS, Milicic A. Reinke S, et al. Cell Rep Med. 2023 Nov 21;4(11):101245. doi: 10.1016/j.xcrm.2023.101245. Epub 2023 Oct 31. Cell Rep Med. 2023. PMID: 37913775 Free PMC article. - The effect of Toll-like receptor agonists on the immunogenicity of MVA-SARS-2-S vaccine after intranasal administration in mice.
Do KTH, Willenzon S, Ristenpart J, Janssen A, Volz A, Sutter G, Förster R, Bošnjak B. Do KTH, et al. Front Cell Infect Microbiol. 2023 Oct 3;13:1259822. doi: 10.3389/fcimb.2023.1259822. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37854858 Free PMC article.
References
- Westgeest KB, de Graaf M, Fourment M, Bestebroer TM, van Beek R, Spronken MI, et al. Genetic evolution of the neuraminidase of influenza A (H3N2) viruses from 1968 to 2009 and its correspondence to haemagglutinin evolution. J Gen Virol. 2012;93(Pt 9):1996–2007. doi: 10.1099/vir.0.043059-0. - DOI - PMC - PubMed
- Wright PF, Neumann G, Kawaoka Y, et al. Orthomyxoviruses. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, et al., editors. Fields virology. 6. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 1186–1243.
- World Health Organization. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003–2007. http://www.who.int/influenza/human_animal_interface/2017_07_25_tableH5N1... Accessed: 25/09/2017. http://www.who.int/influenza/human_animal_interface/2017_07_25_tableH5N1... Accessed 25/09/2017.
- World Health Organization. Human infections with avian influenza A(H5N6) virus. http://www.who.int/csr/don/07-december-2016-ah5n6-china/en/. Accessed: 25/09/2017. Accessed 25/09/2017.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical