Chloroplast Translation: Structural and Functional Organization, Operational Control, and Regulation - PubMed (original) (raw)
Review
Chloroplast Translation: Structural and Functional Organization, Operational Control, and Regulation
Reimo Zoschke et al. Plant Cell. 2018 Apr.
Abstract
Chloroplast translation is essential for cellular viability and plant development. Its positioning at the intersection of organellar RNA and protein metabolism makes it a unique point for the regulation of gene expression in response to internal and external cues. Recently obtained high-resolution structures of plastid ribosomes, the development of approaches allowing genome-wide analyses of chloroplast translation (i.e., ribosome profiling), and the discovery of RNA binding proteins involved in the control of translational activity have greatly increased our understanding of the chloroplast translation process and its regulation. In this review, we provide an overview of the current knowledge of the chloroplast translation machinery, its structure, organization, and function. In addition, we summarize the techniques that are currently available to study chloroplast translation and describe how translational activity is controlled and which _cis_-elements and _trans_-factors are involved. Finally, we discuss how translational control contributes to the regulation of chloroplast gene expression in response to developmental, environmental, and physiological cues. We also illustrate the commonalities and the differences between the chloroplast and bacterial translation machineries and the mechanisms of protein biosynthesis in these two prokaryotic systems.
© 2018 American Society of Plant Biologists. All rights reserved.
Figures
Figure 1.
Common Methods to Analyze Chloroplast Translation. (A) Pulse labeling. Plant cells (chloroplast, large green oval; nucleus, white circle) are fed with radiolabeled cysteine and/or methionine (red dots), which is incorporated together with unlabeled amino acids (black dots) into nascent peptides by translation (for simplicity, only chloroplast ribosomes are shown). Proteins are then isolated, separated by gel electrophoresis, and visualized/quantified by radio-detection methods. (Gel picture kindly provided by Karin Meierhoff.) (B) Polysome analysis. Plant cell lysates are loaded on sucrose gradients (white to black: low to high concentration) to separate RNPs according to their molecular weight by ultracentrifugation. RNA is isolated from gradient fractions and examined by RNA gel blot analysis to determine the ribosome loading of specific mRNAs. (C) Ribosome profiling. Plant cell lysates are treated with nuclease to degrade ribosome-free mRNA sequences. This generates monosomes, whose protected mRNA fragments (ribosome footprints) are subsequently purified. The positions and abundances of the ribosome footprints are determined by next-generation sequencing or microarray hybridization and reflect protein synthesis rates.
Figure 2.
Overview of Internal and External Triggers That Cause Regulatory Adjustments of Translation in Chloroplasts, the Mechanisms That Control Translation, the Coupling of RNA and Protein Metabolism to Chloroplast Translation, and the Localization of the Chloroplast Translation Machinery. Chloroplast translation is regulated in response to internal and external triggers (listed in the upper part). Nucleus-encoded factors are translated in the cytosol (shown in the upper left part) and imported into the chloroplast, where they control and/or regulate chloroplast protein synthesis directly (by altering chloroplast translation activity) or indirectly (by controlling cotranslational chloroplast RNA or protein metabolisms). Chloroplast translation occurs cotranscriptionally (left); however, due to the slow mRNA turnover, the majority of ribosomes act posttranscriptionally (right). RNA binding proteins assist cotranslational RNA processing and/or facilitate translation initiation. Ribosomes initiate and elongate regularly on both processed and unprocessed transcripts, the extent of which seems to mainly depend on the kinetics of the processing events (see text and Figure 3). Many of the factors involved in protein processing, folding, targeting, and assembly act cotranslationally on the nascent polypeptide. See text for details.
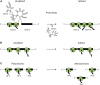
Figure 3.
Ribosomes Translate Unprocessed Chloroplast Transcripts (See Text for Details). (A) Several chloroplast reading frames are interrupted by group II introns. Left: Translating ribosomes cover exon 1 of unspliced atpF, ndhA, ndhB, and ycf3 transcripts (Zoschke et al., 2013a; Alice Barkan, personal communication). Middle and right: Splicing releases the intron and ligates the exons. Consequently, both exons of the spliced transcript are occupied by ribosomes, producing full-length proteins (chain of black dots: nascent polypeptide). (B) Chloroplast transcripts are edited at specific sites by modification of cytosine (C) to uracil (U) residues, often restoring codons for conserved amino acids (change from yellow to white dot in the nascent peptide). Actively translated mRNAs have the same editing status as the total transcriptome (Chotewutmontri and Barkan, 2016), indicating that, in a partially edited transcript pool, unedited transcripts also are translated. (C) Polycistronic chloroplast transcripts often undergo posttranscriptional processing that generates smaller transcript isoforms (represented by the three monocistronic transcripts on the right; RF, reading frame). Often all transcript isoforms are used as translation templates. The extent to which transcript processing may enhance translation efficiency needs to be determined on a case-by-case basis.
Similar articles
- Regulation of chloroplast translation: interactions of RNA elements, RNA-binding proteins and the plastid ribosome.
Manuell A, Beligni MV, Yamaguchi K, Mayfield SP. Manuell A, et al. Biochem Soc Trans. 2004 Aug;32(Pt 4):601-5. doi: 10.1042/BST0320601. Biochem Soc Trans. 2004. PMID: 15270686 - Structure of the chloroplast ribosome: novel domains for translation regulation.
Manuell AL, Quispe J, Mayfield SP. Manuell AL, et al. PLoS Biol. 2007 Aug;5(8):e209. doi: 10.1371/journal.pbio.0050209. PLoS Biol. 2007. PMID: 17683199 Free PMC article. - Dynamics of Chloroplast Translation during Chloroplast Differentiation in Maize.
Chotewutmontri P, Barkan A. Chotewutmontri P, et al. PLoS Genet. 2016 Jul 14;12(7):e1006106. doi: 10.1371/journal.pgen.1006106. eCollection 2016 Jul. PLoS Genet. 2016. PMID: 27414025 Free PMC article. - Chloroplast translation regulation.
Marín-Navarro J, Manuell AL, Wu J, P Mayfield S. Marín-Navarro J, et al. Photosynth Res. 2007 Nov-Dec;94(2-3):359-74. doi: 10.1007/s11120-007-9183-z. Epub 2007 Jul 28. Photosynth Res. 2007. PMID: 17661159 Review. - Chloroplast ribosomes and protein synthesis.
Harris EH, Boynton JE, Gillham NW. Harris EH, et al. Microbiol Rev. 1994 Dec;58(4):700-54. doi: 10.1128/mr.58.4.700-754.1994. Microbiol Rev. 1994. PMID: 7854253 Free PMC article. Review.
Cited by
- From light into shadow: comparative plastomes in Petrocosmea and implications for low light adaptation.
Kan S, Su X, Yang L, Zhou H, Qian M, Zhang W, Li C. Kan S, et al. BMC Plant Biol. 2024 Oct 11;24(1):949. doi: 10.1186/s12870-024-05669-2. BMC Plant Biol. 2024. PMID: 39394065 Free PMC article. - The First Introduction of an Exogenous 5' Untranslated Region for Control of Plastid Transgene Expression in Chlamydomonas reinhardtii.
Abbasi-Vineh MA, Emadpour M. Abbasi-Vineh MA, et al. Mol Biotechnol. 2024 Sep 13. doi: 10.1007/s12033-024-01279-3. Online ahead of print. Mol Biotechnol. 2024. PMID: 39271617 - STIC2 selectively binds ribosome-nascent chain complexes in the cotranslational sorting of Arabidopsis thylakoid proteins.
Stolle DS, Osterhoff L, Treimer P, Lambertz J, Karstens M, Keller JM, Gerlach I, Bischoff A, Dünschede B, Rödiger A, Herrmann C, Baginsky S, Hofmann E, Zoschke R, Armbruster U, Nowaczyk MM, Schünemann D. Stolle DS, et al. EMBO J. 2024 Oct;43(20):4699-4719. doi: 10.1038/s44318-024-00211-4. Epub 2024 Aug 27. EMBO J. 2024. PMID: 39192033 Free PMC article. - Chloroplast Cell-Free Systems from Different Plant Species as a Rapid Prototyping Platform.
Böhm CV, Inckemann R, Burgis M, Baumann J, Brinkmann CK, Lipinska KE, Gilles S, Freudigmann J, Seiler VN, Clark LG, Jewett MC, Voll LM, Niederholtmeyer H. Böhm CV, et al. ACS Synth Biol. 2024 Aug 16;13(8):2412-2424. doi: 10.1021/acssynbio.4c00117. Epub 2024 Jul 19. ACS Synth Biol. 2024. PMID: 39028299 Free PMC article. - Chloroplast Genomes of Vitis flexuosa and Vitis amurensis: Molecular Structure, Phylogenetic, and Comparative Analyses for Wild Plant Conservation.
Kim JE, Kim KM, Kim YS, Chung GY, Che SH, Na CS. Kim JE, et al. Genes (Basel). 2024 Jun 10;15(6):761. doi: 10.3390/genes15060761. Genes (Basel). 2024. PMID: 38927697 Free PMC article.
References
- Akkaya M.S., Breitenberger C.A. (1992). Light regulation of protein synthesis factor EF-G in pea chloroplasts. Plant Mol. Biol. 20: 791–800. - PubMed
- Albrecht V., Ingenfeld A., Apel K. (2006). Characterization of the snowy cotyledon 1 mutant of Arabidopsis thaliana: the impact of chloroplast elongation factor G on chloroplast development and plant vitality. Plant Mol. Biol. 60: 507–518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources