Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer - PubMed (original) (raw)
. 2018 Jun 1;29(6):1437-1444.
doi: 10.1093/annonc/mdy103.
M D Hellmann 2, M Spaziano 3, D Halpenny 4, M Fidelle 1, H Rizvi 5, N Long 4, A J Plodkowski 4, K C Arbour 6, J E Chaft 7, J A Rouche 8, L Zitvogel 9, G Zalcman 10, L Albiges 11, B Escudier 12, B Routy 13
Affiliations
- PMID: 29617710
- PMCID: PMC6354674
- DOI: 10.1093/annonc/mdy103
Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer
L Derosa et al. Ann Oncol. 2018.
Abstract
Background: The composition of gut microbiota affects antitumor immune responses, preclinical and clinical outcome following immune checkpoint inhibitors (ICI) in cancer. Antibiotics (ATB) alter gut microbiota diversity and composition leading to dysbiosis, which may affect effectiveness of ICI.
Patients and methods: We examined patients with advanced renal cell carcinoma (RCC) and non-small-cell lung cancer (NSCLC) treated with anti-programmed cell death ligand-1 mAb monotherapy or combination at two academic institutions. Those receiving ATB within 30 days of beginning ICI were compared with those who did not. Objective response, progression-free survival (PFS) determined by RECIST1.1 and overall survival (OS) were assessed.
Results: Sixteen of 121 (13%) RCC patients and 48 of 239 (20%) NSCLC patients received ATB. The most common ATB were β-lactam or quinolones for pneumonia or urinary tract infections. In RCC patients, ATB compared with no ATB was associated with increased risk of primary progressive disease (PD) (75% versus 22%, P < 0.01), shorter PFS [median 1.9 versus 7.4 months, hazard ratio (HR) 3.1, 95% confidence interval (CI) 1.4-6.9, P < 0.01], and shorter OS (median 17.3 versus 30.6 months, HR 3.5, 95% CI 1.1-10.8, P = 0.03). In NSCLC patients, ATB was associated with similar rates of primary PD (52% versus 43%, P = 0.26) but decreased PFS (median 1.9 versus 3.8 months, HR 1.5, 95% CI 1.0-2.2, P = 0.03) and OS (median 7.9 versus 24.6 months, HR 4.4, 95% CI 2.6-7.7, P < 0.01). In multivariate analyses, the impact of ATB remained significant for PFS in RCC and for OS in NSCLC.
Conclusion: ATB were associated with reduced clinical benefit from ICI in RCC and NSCLC. Modulatation of ATB-related dysbiosis and gut microbiota composition may be a strategy to improve clinical outcomes with ICI.
Figures
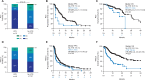
Figure 1.
Best overall response (A), progression-free survival (PFS) (B), and overall survival (OS) (C) in patients with RCC treated with ICI, stratified by use of ATB within 30 days of initiating ICI. Best overall response (D), PFS (E), and OS (F) in patients with NSCLC treated with ICI, stratified by use of ATB within 30 days of initiating ICI._P_-values calculated with chi-squared and log-rank tests.
Figure 2.
Subgroup analyses of independent prognostic factors for PFS (A and B) and OS (C and D) stratification in RCC and NSCLC, respectively. _P_-value for interaction calculated with Cox proportional hazards model.
Comment in
- Further evidence to support judicious use of antibiotics in patients with cancer.
Luke JJ, Pal SK. Luke JJ, et al. Ann Oncol. 2018 Jun 1;29(6):1349-1351. doi: 10.1093/annonc/mdy153. Ann Oncol. 2018. PMID: 29688263 Free PMC article. No abstract available.
Similar articles
- Predictive impact of antibiotics in patients with advanced non small-cell lung cancer receiving immune checkpoint inhibitors : Antibiotics immune checkpoint inhibitors in advanced NSCLC.
Schett A, Rothschild SI, Curioni-Fontecedro A, Krähenbühl S, Früh M, Schmid S, Driessen C, Joerger M. Schett A, et al. Cancer Chemother Pharmacol. 2020 Jan;85(1):121-131. doi: 10.1007/s00280-019-03993-1. Epub 2019 Nov 19. Cancer Chemother Pharmacol. 2020. PMID: 31745593 - The effects of antibiotics on the efficacy of immune checkpoint inhibitors in patients with non-small-cell lung cancer differ based on PD-L1 expression.
Ochi N, Ichihara E, Takigawa N, Harada D, Inoue K, Shibayama T, Hosokawa S, Kishino D, Harita S, Oda N, Hara N, Hotta K, Maeda Y, Kiura K. Ochi N, et al. Eur J Cancer. 2021 May;149:73-81. doi: 10.1016/j.ejca.2021.02.040. Epub 2021 Apr 7. Eur J Cancer. 2021. PMID: 33838391 - Impact of Antibiotics and Proton Pump Inhibitors on Efficacy and Tolerance of Anti-PD-1 Immune Checkpoint Inhibitors.
Giordan Q, Salleron J, Vallance C, Moriana C, Clement-Duchene C. Giordan Q, et al. Front Immunol. 2021 Oct 27;12:716317. doi: 10.3389/fimmu.2021.716317. eCollection 2021. Front Immunol. 2021. PMID: 34777340 Free PMC article. - The negative impact of antibiotics on outcomes in cancer patients treated with immunotherapy: a new independent prognostic factor?
Elkrief A, Derosa L, Kroemer G, Zitvogel L, Routy B. Elkrief A, et al. Ann Oncol. 2019 Oct 1;30(10):1572-1579. doi: 10.1093/annonc/mdz206. Ann Oncol. 2019. PMID: 31268133 Review. - The Gut Microbiome from a Biomarker to a Novel Therapeutic Strategy for Immunotherapy Response in Patients with Lung Cancer.
Duttagupta S, Hakozaki T, Routy B, Messaoudene M. Duttagupta S, et al. Curr Oncol. 2023 Oct 24;30(11):9406-9427. doi: 10.3390/curroncol30110681. Curr Oncol. 2023. PMID: 37999101 Free PMC article. Review.
Cited by
- Effect of antibiotic drug use on outcome and therapy-related toxicity in patients with glioblastoma-A retrospective cohort study.
Götz L, Ansafi T, Gerken M, Klinkhammer-Schalke M, Fischl A, Riemenschneider MJ, Proescholdt M, Bumes E, Kölbl O, Schmidt NO, Linker R, Hau P, Haedenkamp TM. Götz L, et al. Neurooncol Adv. 2024 Oct 4;6(1):vdae170. doi: 10.1093/noajnl/vdae170. eCollection 2024 Jan-Dec. Neurooncol Adv. 2024. PMID: 39493414 Free PMC article. - Predictive biomarkers for immune checkpoint inhibitors therapy in lung cancer.
Yao J, Lin X, Zhang X, Xie M, Ma X, Bao X, Song J, Liang Y, Wang Q, Xue X. Yao J, et al. Hum Vaccin Immunother. 2024 Dec 31;20(1):2406063. doi: 10.1080/21645515.2024.2406063. Epub 2024 Oct 16. Hum Vaccin Immunother. 2024. PMID: 39415535 Free PMC article. Review. - Salivary biomarkers: a promising approach for predicting immunotherapy response in head and neck cancers.
Nejat Dehkordi A, Maddahi M, Vafa P, Ebrahimi N, Aref AR. Nejat Dehkordi A, et al. Clin Transl Oncol. 2024 Oct 8. doi: 10.1007/s12094-024-03742-8. Online ahead of print. Clin Transl Oncol. 2024. PMID: 39377974 Review. - Interaction of the Gut Microbiome With Cancer Treatment.
Barrenechea PM. Barrenechea PM. J Adv Pract Oncol. 2024 Jul;15(5):311-319. doi: 10.6004/jadpro.2024.15.5.3. Epub 2024 Jul 1. J Adv Pract Oncol. 2024. PMID: 39328379 Free PMC article. Review. - Pharmacomicrobiomics in precision cancer therapy: bench to bedside.
Le Ngoc K, Pham TTH, Nguyen TK, Huong PT. Le Ngoc K, et al. Front Immunol. 2024 Sep 9;15:1428420. doi: 10.3389/fimmu.2024.1428420. eCollection 2024. Front Immunol. 2024. PMID: 39315107 Free PMC article. Review.
References
- Escudier B, Sharma P, McDermott DF. et al. CheckMate 025 randomized phase 3 study: outcomes by key baseline factors and prior therapy for nivolumab versus everolimus in advanced renal cell carcinoma. Eur Urol 2017. Mar 3 [Epub ahead of print], doi: 10.1016/j.eururo.2017.02.010. - PubMed
- Reck M, Rodríguez-Abreu D, Robinson AG. et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375(19): 1823–1833. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical