Diabetes-Specific Formulae Versus Standard Formulae as Enteral Nutrition to Treat Hyperglycemia in Critically Ill Patients: Protocol for a Randomized Controlled Feasibility Trial - PubMed (original) (raw)
Diabetes-Specific Formulae Versus Standard Formulae as Enteral Nutrition to Treat Hyperglycemia in Critically Ill Patients: Protocol for a Randomized Controlled Feasibility Trial
Ra'eesa Doola et al. JMIR Res Protoc. 2018.
Abstract
Background: During critical illness, hyperglycemia is prevalent and is associated with adverse outcomes. While treating hyperglycemia with insulin reduces morbidity and mortality, it increases glycemic variability and hypoglycemia risk, both of which have been associated with an increase in mortality. Therefore, other interventions which improve glycemic control, without these complications should be explored. Nutrition forms part of standard care, but the carbohydrate load of these formulations has the potential to exacerbate hyperglycemia. Specific diabetic-formulae with a lesser proportion of carbohydrate are available, and these formulae are postulated to limit glycemic excursions and reduce patients' requirements for exogenous insulin.
Objective: The primary outcome of this prospective, blinded, single center, randomized controlled trial is to determine whether a diabetes-specific formula reduces exogenous insulin administration. Key secondary outcomes include the feasibility of study processes as well as glycemic variability.
Methods: Critically ill patients will be eligible if insulin is administered whilst receiving exclusively liquid enteral nutrition. Participants will be randomized to receive a control formula, or a diabetes-specific, low glycemic index, low in carbohydrate study formula. Additionally, a third group of patients will receive a second diabetes-specific, low glycemic index study formula, as part of a sub-study to evaluate its effect on biomarkers. This intervention group (n=12) will form part of recruitment to a nested cohort study with blood and urine samples collected at randomization and 48 hours later for the first 12 participants in each group with a secondary objective of exploring the metabolic implications of a change in nutrition formula. Data on relevant medication and infusions, nutrition provision and glucose control will be collected to a maximum of 48 hours post randomization. Baseline patient characteristics and anthropometric measures will be recorded. A 28-day phone follow-up will explore weight and appetite changes as well as blood glucose control pre and post intensive care unit (ICU) discharge.
Results: Recruitment commenced in February 2015 with an estimated completion date for data collection by May 2018. Results are expected to be available late 2018.
Conclusions: This feasibility study of the effect of diabetes-specific formulae on the administration of insulin in critically ill patients and will inform the design of a larger, multi-center trial.
Trial registration: Australian New Zealand Clinical Trial Registry (ANZCTR):12614000166673; https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12614000166673 (Archived by WebCite at http://www.webcitation.org/6xs0phrVu).
Keywords: enteral formula; glucose; glycemic control; low carbohydrate; nutrition; tube formula.
©Ra'eesa Doola, Alwyn S Todd, Josephine M Forbes, Adam M Deane, Jeffrey J Presneill, David J Sturgess. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 09.04.2018.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Figure 1
Blinding of formulae.
Figure 2
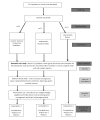
Study flow diagram of recruitment, randomization and study conduct. Once consented patients will be randomized on a 1:1:1 ratio until there are 12 patients who have complete blood and urine sample sets from each group to form the biomarker sub-study. Thereafter the study will proceed with two arms as indicated. Finalized patient numbers have not been provided as this is a feasibility study.
Similar articles
- Diabetes-specific enteral nutrition formula in hyperglycemic, mechanically ventilated, critically ill patients: a prospective, open-label, blind-randomized, multicenter study.
Mesejo A, Montejo-González JC, Vaquerizo-Alonso C, Lobo-Tamer G, Zabarte-Martinez M, Herrero-Meseguer JI, Acosta-Escribano J, Blesa-Malpica A, Martinez-Lozano F. Mesejo A, et al. Crit Care. 2015 Nov 9;19:390. doi: 10.1186/s13054-015-1108-1. Crit Care. 2015. PMID: 26549276 Free PMC article. Clinical Trial. - The effect of a low carbohydrate formula on glycaemia in critically ill enterally-fed adult patients with hyperglycaemia: A blinded randomised feasibility trial.
Doola R, Deane AM, Tolcher DM, Presneill JJ, Barrett HL, Forbes JM, Todd AS, Okano S, Sturgess DJ. Doola R, et al. Clin Nutr ESPEN. 2019 Jun;31:80-87. doi: 10.1016/j.clnesp.2019.02.013. Epub 2019 Mar 11. Clin Nutr ESPEN. 2019. PMID: 31060838 Clinical Trial. - Glycemic Effects of a Low-Carbohydrate Enteral Formula Compared With an Enteral Formula of Standard Composition in Critically Ill Patients: An Open-Label Randomized Controlled Clinical Trial.
van Steen SC, Rijkenberg S, Sechterberger MK, DeVries JH, van der Voort PHJ. van Steen SC, et al. JPEN J Parenter Enteral Nutr. 2018 Aug;42(6):1035-1045. doi: 10.1002/jpen.1045. Epub 2017 Dec 27. JPEN J Parenter Enteral Nutr. 2018. PMID: 30133840 Clinical Trial. - [Guidelines for specialized nutritional and metabolic support in the critically-ill patient. Update. Consensus of the Spanish Society of Intensive Care Medicine and Coronary Units-Spanish Society of Parenteral and Enteral Nutrition (SEMICYUC-SENPE): hyperglycemia and diabetes mellitus].
Vaquerizo Alonso C, Grau Carmona T, Juan Díaz M; Spanish Society of Intensive Care Medicine and Coronary Units-Spanish Society of Parenteral and Enteral Nutrition (SEMICYUC-SENPE). Vaquerizo Alonso C, et al. Med Intensiva. 2011 Nov;35 Suppl 1:48-52. doi: 10.1016/S0210-5691(11)70010-9. Med Intensiva. 2011. PMID: 22309753 Spanish. - Glucose control in the intensive care unit.
Fahy BG, Sheehy AM, Coursin DB. Fahy BG, et al. Crit Care Med. 2009 May;37(5):1769-76. doi: 10.1097/CCM.0b013e3181a19ceb. Crit Care Med. 2009. PMID: 19325461 Review.
Cited by
- Effect of Oral Nutritional Supplements with Sucromalt and Isomaltulose versus Standard Formula on Glycaemic Index, Entero-Insular Axis Peptides and Subjective Appetite in Patients with Type 2 Diabetes: A Randomised Cross-Over Study.
Angarita Dávila L, Bermúdez V, Aparicio D, Céspedes V, Escobar MC, Durán-Agüero S, Cisternas S, de Assis Costa J, Rojas-Gómez D, Reyna N, López-Miranda J. Angarita Dávila L, et al. Nutrients. 2019 Jun 28;11(7):1477. doi: 10.3390/nu11071477. Nutrients. 2019. PMID: 31261732 Free PMC article. Clinical Trial. - Nutritional Values of Teff (Eragrostis tef) in Diabetic Patients: Narrative Review.
Habte ML, Beyene EA, Feyisa TO, Admasu FT, Tilahun A, Diribsa GC. Habte ML, et al. Diabetes Metab Syndr Obes. 2022 Aug 22;15:2599-2606. doi: 10.2147/DMSO.S366958. eCollection 2022. Diabetes Metab Syndr Obes. 2022. PMID: 36035517 Free PMC article. Review. - The Effect of Oral Nutritional Formula With Three Different Proteins on Type 2 Diabetes Mellitus in vivo.
Jia Y, Leng Y, Cruz ALP, Bao CL, Bao B, Wu W, Wang P, Ma M. Jia Y, et al. Front Nutr. 2021 Sep 21;8:680700. doi: 10.3389/fnut.2021.680700. eCollection 2021. Front Nutr. 2021. PMID: 34621771 Free PMC article. - Glycemic Control in Diabetic Patients Receiving a Diabetes-Specific Nutritional Enteral Formula: A Case Series in Home Care Settings.
Pantanetti P, Cangelosi G, Sguanci M, Morales Palomares S, Nguyen CTT, Morresi G, Mancin S, Petrelli F. Pantanetti P, et al. Nutrients. 2024 Aug 7;16(16):2602. doi: 10.3390/nu16162602. Nutrients. 2024. PMID: 39203739 Free PMC article.
References
- Wischmeyer PE. The evolution of nutrition in critical care: how much, how soon? Crit Care. 2013;17 Suppl 1:S7. doi: 10.1186/cc11505. https://ccforum.com/1364-8535/17/S1/S7 cc11505 - DOI - PMC - PubMed
- Al-Tarifi A, Abou-Shala N, Tamim HM, Rishu AH, Arabi YM. What is the optimal blood glucose target in critically ill patients? A nested cohort study. Ann Thorac Med. 2011 Oct;6(4):207–11. doi: 10.4103/1817-1737.84774. http://www.thoracicmedicine.org/article.asp?issn=1817-1737;year=2011;vol... ATM-6-207 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources