Helicobacter suis binding to carbohydrates on human and porcine gastric mucins and glycolipids occurs via two modes - PubMed (original) (raw)
. 2018 Dec 31;9(1):898-918.
doi: 10.1080/21505594.2018.1460979.
Barbara Adamczyk 1, John Benktander 1, Bram Flahou 2, Emma C Skoog 1, János Tamás Padra 1, Annemieke Smet 2 3, Chunsheng Jin 1, Richard Ducatelle 2, Tore Samuelsson 1, Freddy Haesebrouck 2, Niclas G Karlsson 1, Susann Teneberg 1, Sara K Lindén 1
Affiliations
- PMID: 29638186
- PMCID: PMC5955484
- DOI: 10.1080/21505594.2018.1460979
Helicobacter suis binding to carbohydrates on human and porcine gastric mucins and glycolipids occurs via two modes
Médea Padra et al. Virulence. 2018.
Abstract
Helicobacter suis colonizes the stomach of most pigs and is the most prevalent non-Helicobacter pylori Helicobacter species found in the human stomach. In the human host, H. suis contributes to the development of chronic gastritis, peptic ulcer disease and MALT lymphoma, whereas in pigs it is associated with gastritis, decreased growth and ulcers. Here, we demonstrate that the level of H. pylori and H. suis binding to human and pig gastric mucins varies between individuals with species dependent specificity. The binding optimum of H. pylori is at neutral pH whereas that of H. suis has an acidic pH optimum, and the mucins that H. pylori bind to are different than those that H. suis bind to. Mass spectrometric analysis of mucin O-glycans from the porcine mucin showed that individual variation in binding is reflected by a difference in glycosylation; of 109 oligosaccharide structures identified, only 14 were present in all examined samples. H. suis binding to mucins correlated with glycans containing sulfate, sialic acid and terminal galactose. Among the glycolipids present in pig stomach, binding to lactotetraosylceramide (Galβ3GlcNAcβ3Galβ4Glcβ1Cer) was identified, and adhesion to Galβ3GlcNAcβ3Galβ4Glc at both acidic and neutral pH was confirmed using other glycoconjugates. Together with that H. suis bound to DNA (used as a proxy for acidic charge), we conclude that H. suis has two binding modes: one to glycans terminating with Galβ3GlcNAc, and one to negatively charged structures. Identification of the glycan structures H. suis interacts with can contribute to development of therapeutic strategies alternative to antibiotics.
Keywords: Helicobacter pylori; Helicobacter suis; bacterial adhesion; glycolipid; glycosylation; host–pathogen interactions; mucin.
Figures
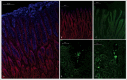
Figure 1.
Spatial distribution of MUC5AC and H. suis in porcine gastric specimens. A. Porcine gastric tissue fixed with Carnoy´s fixative to retain the mucus layer was stained with a fluorescently labelled antibody against MUC5AC (blue), demonstrating a thick mucus layer above the epithelium. Nuclei and cytoplasm were stained with HCS CellMask™ (red). B. Negative control for MUC5AC staining (i.e. no MUC5AC antibody was added) with nuclei and cytoplasm outlined with HCS CellMask™ (red). C, D, E. Fluorescent in situ hybridization using an H. suis specific probe on H. suis –free (C) and infected (D, E) porcine gastric tissue sections. In these images, mucus is not visible, but the tissue is outlined by dull green auto fluorescence. H. suis (bright green color) was detected in the mucus layer in the gastric pits (D). Of the H. suis present in the tissue, most were found in the lamina propria and/or associated with the (fried egg shaped) parietal cells (E).
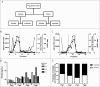
Figure 2.
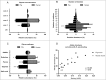
Isolation, solubility and density of porcine gastric mucins. A. Each specimen was separated into four types of mucin samples based on their tissue location and solubility in GuHCl. Mucins were then isolated from these samples using two steps of isopycnic CsCl density-gradient centrifugation. B. Fractions from the first density-gradient centrifugation (4 M GuHCl/1.39 g/mL) were analyzed for carbohydrate (▴), MUC5AC (▪), and DNA (─). The vertical dashed lines indicate how the mucin containing fractions were pooled for further purification in the second density-gradient centrifugation. C. Fractions from the second density-gradient centrifugation (0.5 M GuHCl/1.50 g/mL) were analyzed for carbohydrate (▴), MUC5AC (▪), and DNA (─). The vertical dashed lines indicate how the mucin containing fractions were pooled for further experiments. D. The density of the pig gastric mucins ranged from 1.38 to 1.47 g/mL. E. The proportion of mucins that were insoluble in GuHCl, was higher than the soluble (Mann-Whitney U-test: p ≤ 0.05). Abbreviations: SS: surface soluble, SI: surface insoluble, GS: gland soluble, GI: gland insoluble.
Figure 3.
Effect of pH and mucin type on H. suis binding to pig gastric mucins. A. pH dependence of H. suis binding to mucins. Results are shown as mean ± SEM of bacterial binding after subtraction of background signal at each pH. ** and *** indicate p ≤ 0.01 and 0.001, respectively, in an unpaired two sided t-test comparing the binding to the negative control background at each pH. This assay was performed on four mucin samples, with similar results, ranging between the pronounced pH dependence shown with mucin 1 to the flatter curve seen with mucin 2. B. Effect of mucin type (surface (•), gland (○), soluble and insoluble) on H. suis binding at pH 2. Results are expressed as medians with interquartile ranges. ** indicates p ≤ 0.01 (Mann-Whitney U-test).
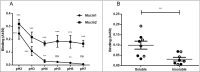
Figure 4.
H. suis binding to pig and human gastric mucins. A, B, C. H. suis strains HS1 and HS5 binding to pig mucin groups (SS – surface soluble, SI – surface insoluble, GS – gland soluble, GI – gland insoluble) isolated from the stomach of four pigs (P1-4). D, E, F. Binding of H. suis strains HS1 and HS5 to human gastric mucins isolated from five human patients (H1-H5). G, H, I. Binding of H. pylori strain J99 wild type and its isogenic ΔbabA/ΔsabA deletion mutant to human gastric mucins. Results are shown as mean ± SEM of bacterial binding after subtraction of background signal at each pH. *, ** and *** indicate p ≤ 0.05, 0.01 and 0.001, respectively, One Way ANOVA, Dunnett´s post hoc test.
Figure 5.
Comparison of binding of four H. suis strains to pig and human gastric mucins. H. suis binding at pH 2 (A), pH 4 (B) and pH 7 (C) to a human mucin, a pig mucin with high H. suis binding ability and a pig mucin with low H. suis binding ability.
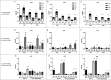
Figure 6.
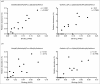
Porcine and human mucin _O_-glycosylation. A. Relative abundance of pig and human structures containing core 1–4 and sialyl-Tn. B. Size distribution (number of carbohydrate residues/glycan) of pig and human mucin _O_-glycans. C. Relative abundance of terminal glycan residues and sulfation on pig (n = 3, 12 subfractions) and human (n = 5) mucin glycans. Stars indicate statistically significant difference between pig and human mucin glycans, *, ** and *** indicate p ≤ 0.05, 0.01 and 0.001, respectively, Two-way ANOVA. D. Scatter plot of the amplitude of H. suis binding to mucins at pH 2 against their relative abundance of acidic glycan structures among pig (mainly sulfated structures, black) and human (mainly sialylated structures, grey). The r in the figures denotes the Pearson correlation coefficient, pooled for the human and pig data (pig mucins only: r = 0.85, human samples alone are too few to perform correlation analysis on).
Figure 7.
Scatter plots between H. suis adhesion and the relative abundance of the two structures that were associated with binding at both pH 2 and 7. The r in the figures denotes the Pearson correlation coefficient.
Figure 8.
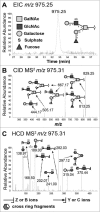
Identification of the m/z 975.25 structure (Galβ4(6S)GlcNAcβ6(Fucα2Galβ3)GalNAcol) from porcine gastric mucin _O_-glycans by LC-MS/MS. A. Extracted ion chromatogram (EIC) of m/z 975.25 [M-H]− B. Collision-induced dissociation (CID)-based MS/MS fragmentation spectra with annotated fragments C. Higher-energy collisional dissociation (HCD)-based MS/MS low mass region (m/z 110–390) showing fragments specific for blood group H on C3 arm and a type 2 LacNAc on C6 with 6-linked sulfate on GlcNAc. * denotes background ions. The Consortium for Functional Glycomics cartoons were used to represent _O_-linked glycan structures.
Figure 9.
Characterization of H. suis binding glycosphingolipids from pig stomach. A. Thin-layer chromatogram after detection with anisaldehyde B. Autoradiogram obtained by binding of 35S-labeled H. suis (HS5) at pH 7.2. C. Autoradiogram obtained by binding of 35S-labeled H. pylori strain J99 wt at pH 7.2. The glycosphingolipids were separated on aluminum-backed silica gel plates, using chloroform/methanol/water 60:35:8 (by volume) as solvent system. Lanes 1–4 were total non-acid glycosphingolipids isolated from the stomach of four individual pigs, 40 µg/lane. D-G. LC-ESI/MS of the oligosaccharides obtained by digestion of the non-acid glycosphingolipid fraction of pig stomach (shown in chart A, lane 2) with Rhodococcus endoglycoceramidase II. D. Base peak chromatogram from LC-ESI/MS of the oligosaccharides derived from the non-acid glycosphingolipid fraction of pig stomach. E. MS2 spectrum of the ion at m/z 706 (retention time 14.2 min). F. MS2 spectrum of the ion at m/z 706 (retention time 18.3 min). G. MS2 spectrum of the ion at m/z 706 (retention time 18.5 min). H. Interpretation formulas showing the deduced oligosaccharide structures. Gb3, Galα4Galβ4Glc; Gb4, GalNAcβ3Galα4Galβ4Glc; A6-1, GalNAcα3(Fucα2)Galβ3GlcNAcβ3Galβ4Glc; Ley-6, Fucα2Galβ4(Fucα3)GlcNAcβ3Galβ4Glc; H5-1, Fucα2Galβ3GlcNAcβ3Galβ4Glc; L4, Galβ3GlcNAcβ3Galβ4Glc; nL4, Galβ4GlcNAcβ3Galβ4Glc; H5-2, Fucα2Galβ4GlcNAcβ3Galβ4Glc; Galα3-nLc4, Galα3Galβ4GlcNAcβ3Galβ4Glc; x2, GalNAcβ3Galβ4GlcNAcβ3Galβ4Glc; Galα3-nLc4, Galα3Galβ4GlcNAcβ3Galβ4GlcNAcβ3Galβ4Glc; GbA, GalNAcα3(Fucα2Galβ3GalNAcβ3Galα4Galβ4Glc.
Figure 10.
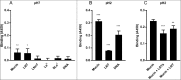
H. suis binding to synthetic glycoconjugates and inhibition of H. suis mucin binding. A. H. suis (HS1) binding to pig gastric mucin, LNT, LNnT, Leb, Slex (glycans conjugated to HSA) and DNA at pH 7. B. H. suis binding to pig gastric mucin, LNT conjugated to HSA and DNA (used as a marker for acidic charge without structural resemblance of mucins) at pH 2. C. Binding of H. suis to mucins was inhibited by pre-treatment of the bacteria with LNT and LSTa. To confirm the specificity of the inhibition, the experiment was also performed with monosaccharides that are part of the structures that inhibited binding (glucose, galactose, _N_-acetyl-glucosamine) and also with unrelated monosaccharides (fucose, _N_-acetyl-galactosamine). None of these structures interfered with the binding of the bacteria to the mucin (data not shown). Data are shown after subtracting background control for each pH. * indicates p ≤ 0.05, ** p ≤ 0.01 and *** p ≤ 0.001, One-way ANOVA, Dunnett´s post hoc test.
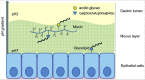
Figure 11.
Schematic representation of the two binding modes of H. suis. H. suis binds to Galβ3GlcNAcβ3Galβ4Glcβ1 at both neutral and acidic pH and to negatively charged glycan structures at acidic pH. Galβ3GlcNAcβ3Galβ4Glcβ1 is present both on glycolipids at the epithelial surface where pH is close to neutral and on secreted mucins in the mucus layer where pH range from neutral to acidic, and H. suis binding can thus occur to this structure both when present on glycolipids and on mucins. H. suis binding to negatively charged glycan structures at acidic pH is likely to mainly occur to mucin glycans and shed DNA at a distance from the epithelial surface where the pH is acidic. Potentially binding to these structures could also occur after tissue invasion, i.e. at neutral pH when H. suis is present in the lamina propria and at acidic pH inside parietal cell canaliculi, however, the distribution of these structures in lamina propria and parietal cells is unknown.
Similar articles
- Adhesion of Helicobacter Species to the Human Gastric Mucosa: A Deep Look Into Glycans Role.
Matos R, Amorim I, Magalhães A, Haesebrouck F, Gärtner F, Reis CA. Matos R, et al. Front Mol Biosci. 2021 May 7;8:656439. doi: 10.3389/fmolb.2021.656439. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026832 Free PMC article. Review. - Helicobacter suis infection alters glycosylation and decreases the pathogen growth inhibiting effect and binding avidity of gastric mucins.
Padra M, Adamczyk B, Flahou B, Erhardsson M, Chahal G, Smet A, Jin C, Thorell A, Ducatelle R, Haesebrouck F, Karlsson NG, Lindén SK. Padra M, et al. Mucosal Immunol. 2019 May;12(3):784-794. doi: 10.1038/s41385-019-0154-4. Epub 2019 Mar 7. Mucosal Immunol. 2019. PMID: 30846831 - Evidence for a primate origin of zoonotic Helicobacter suis colonizing domesticated pigs.
Flahou B, Rossi M, Bakker J, Langermans JA, Heuvelman E, Solnick JV, Martin ME, O'Rourke J, Ngoan LD, Hoa NX, Nakamura M, Øverby A, Matsui H, Ota H, Matsumoto T, Foss DL, Kopta LA, Omotosho O, Franciosini MP, Casagrande Proietti P, Guo A, Liu H, Borilova G, Bracarense AP, Lindén SK, De Bruyckere S, Zhang G, De Witte C, Smet A, Pasmans F, Ducatelle R, Corander J, Haesebrouck F. Flahou B, et al. ISME J. 2018 Jan;12(1):77-86. doi: 10.1038/ismej.2017.145. Epub 2017 Sep 8. ISME J. 2018. PMID: 28885626 Free PMC article. - A Complex Connection Between the Diversity of Human Gastric Mucin O-Glycans, Helicobacter pylori Binding, Helicobacter Infection and Fucosylation.
Chahal G, Padra M, Erhardsson M, Jin C, Quintana-Hayashi M, Venkatakrishnan V, Padra JT, Stenbäck H, Thorell A, Karlsson NG, Lindén SK. Chahal G, et al. Mol Cell Proteomics. 2022 Nov;21(11):100421. doi: 10.1016/j.mcpro.2022.100421. Epub 2022 Sep 29. Mol Cell Proteomics. 2022. PMID: 36182101 Free PMC article. - Gastric Helicobacter species associated with dogs, cats and pigs: significance for public and animal health.
Taillieu E, Chiers K, Amorim I, Gärtner F, Maes D, Van Steenkiste C, Haesebrouck F. Taillieu E, et al. Vet Res. 2022 Jun 13;53(1):42. doi: 10.1186/s13567-022-01059-4. Vet Res. 2022. PMID: 35692057 Free PMC article. Review.
Cited by
- Role of mucus-bacteria interactions in Enterotoxigenic Escherichia coli (ETEC) H10407 virulence and interplay with human microbiome.
Sauvaitre T, Van Landuyt J, Durif C, Roussel C, Sivignon A, Chalancon S, Uriot O, Van Herreweghen F, Van de Wiele T, Etienne-Mesmin L, Blanquet-Diot S. Sauvaitre T, et al. NPJ Biofilms Microbiomes. 2022 Oct 20;8(1):86. doi: 10.1038/s41522-022-00344-6. NPJ Biofilms Microbiomes. 2022. PMID: 36266277 Free PMC article. - The prevalence of porcine gastric ulcer and Helicobacter suis in Taiwan.
Lin PJ, Liao CW, Chiang HH, Lo DY, Kuo HC, Wu CF. Lin PJ, et al. J Vet Med Sci. 2024 Jun 19;86(6):670-676. doi: 10.1292/jvms.23-0403. Epub 2024 May 1. J Vet Med Sci. 2024. PMID: 38692859 Free PMC article. - Adhesion of Helicobacter Species to the Human Gastric Mucosa: A Deep Look Into Glycans Role.
Matos R, Amorim I, Magalhães A, Haesebrouck F, Gärtner F, Reis CA. Matos R, et al. Front Mol Biosci. 2021 May 7;8:656439. doi: 10.3389/fmolb.2021.656439. eCollection 2021. Front Mol Biosci. 2021. PMID: 34026832 Free PMC article. Review. - How Does Airway Surface Liquid Composition Vary in Different Pulmonary Diseases, and How Can We Use This Knowledge to Model Microbial Infections?
Walsh D, Bevan J, Harrison F. Walsh D, et al. Microorganisms. 2024 Apr 3;12(4):732. doi: 10.3390/microorganisms12040732. Microorganisms. 2024. PMID: 38674677 Free PMC article. Review. - Fish pathogen binding to mucins from Atlantic salmon and Arctic char differs in avidity and specificity and is modulated by fluid velocity.
Padra JT, Murugan AVM, Sundell K, Sundh H, Benktander J, Lindén SK. Padra JT, et al. PLoS One. 2019 May 24;14(5):e0215583. doi: 10.1371/journal.pone.0215583. eCollection 2019. PLoS One. 2019. PMID: 31125340 Free PMC article.
References
- Barbosa AJ, Silva JC, Nogueira AM, Paulino Junior E, Miranda CR. Higher incidence of Gastrospirillum sp. in swine with gastric ulcer of the pars oesophagea. Veterinary pathology 1995; 32:134–9. - PubMed
- De Bruyne E Flahou B, Chiers K, Meyns T, Kumar S, Vermoote M, Pasmans F, Millet S, Dewulf J, Haesebrouck F, et al. . An experimental Helicobacter suis infection causes gastritis and reduced daily weight gain in pigs. Veterinary microbiology 2012; 160:449–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by the Cancerfonden (160716), Cancerfonden (2014/682), Jeanssons Stiftelser (2009), Ghent University (BOF14/GOA/010), Ghent University (GOA01G0040),richard julin foundation (2015juli44247), FP7 Initial Training Networks (316929), Ragnar Söderbergs stiftelse (M36/11), Sahlgrenska Universitetssjukhuset (160631), Wilhelm och Martina Lundgrens Stiftelserna (2015-0699), Wilhelm och Martina Lundgrens Stiftelserna (2015-0688), Svenska Forskningsrådet Formas (221-2011-1036) and Svenska Forskningsrådet Formas (221-2013-590).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical