Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair - PubMed (original) (raw)
doi: 10.1186/s13287-018-0850-0.
Agnese Gugliandolo [ 2](#full-view-affiliation-2 "IRCCS Centro Neurolesi "Bonino Pulejo", Messina, Italy."), Paolo Cardelli [ 1](#full-view-affiliation-1 "Department of Medical, Oral and Biotechnological Sciences, University "G. d'Annunzio", Chieti, Italy."), Ilaria Merciaro [ 1](#full-view-affiliation-1 "Department of Medical, Oral and Biotechnological Sciences, University "G. d'Annunzio", Chieti, Italy."), Valeria Ettorre [ 3](#full-view-affiliation-3 "Department of Pharmacy, University "G. d'Annunzio", Chieti, Italy."), Tonino Traini [ 1](#full-view-affiliation-1 "Department of Medical, Oral and Biotechnological Sciences, University "G. d'Annunzio", Chieti, Italy."), Rossella Bedini 4, Domenico Scionti [ 2](#full-view-affiliation-2 "IRCCS Centro Neurolesi "Bonino Pulejo", Messina, Italy."), Alessia Bramanti [ 2](#full-view-affiliation-2 "IRCCS Centro Neurolesi "Bonino Pulejo", Messina, Italy.") [ 5](#full-view-affiliation-5 "Institute of Applied Science and Intelligent Systems "ISASI Eduardo Caianiello", CNR, Messina, Italy."), Antonio Nanci 6, Sergio Caputi [ 1](#full-view-affiliation-1 "Department of Medical, Oral and Biotechnological Sciences, University "G. d'Annunzio", Chieti, Italy."), Antonella Fontana [ 3](#full-view-affiliation-3 "Department of Pharmacy, University "G. d'Annunzio", Chieti, Italy."), Emanuela Mazzon [ 2](#full-view-affiliation-2 "IRCCS Centro Neurolesi "Bonino Pulejo", Messina, Italy."), Oriana Trubiani [ 7](#full-view-affiliation-7 "Department of Medical, Oral and Biotechnological Sciences, University "G. d'Annunzio", Via dei Vestini, 66100, Chieti, Italy. trubiani@unich.it.")
Affiliations
- PMID: 29653587
- PMCID: PMC5899396
- DOI: 10.1186/s13287-018-0850-0
Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair
Francesca Diomede et al. Stem Cell Res Ther. 2018.
Abstract
Background: The role of bone tissue engineering in the field of regenerative medicine has been a main research topic over the past few years. There has been much interest in the use of three-dimensional (3D) engineered scaffolds (PLA) complexed with human gingival mesenchymal stem cells (hGMSCs) as a new therapeutic strategy to improve bone tissue regeneration. These devices can mimic a more favorable endogenous microenvironment for cells in vivo by providing 3D substrates which are able to support cell survival, proliferation and differentiation. The present study evaluated the in vitro and in vivo capability of bone defect regeneration of 3D PLA, hGMSCs, extracellular vesicles (EVs), or polyethyleneimine (PEI)-engineered EVs (PEI-EVs) in the following experimental groups: 3D-PLA, 3D-PLA + hGMSCs, 3D-PLA + EVs, 3D-PLA + EVs + hGMSCs, 3D-PLA + PEI-EVs, 3D-PLA + PEI-EVs + hGMSCs.
Methods: The structural parameters of the scaffold were evaluated using both scanning electron microscopy and nondestructive microcomputed tomography. Nanotopographic surface features were investigated by means of atomic force microscopy. Scaffolds showed a statistically significant mass loss along the 112-day evaluation.
Results: Our in vitro results revealed that both 3D-PLA + EVs + hGMSCs and 3D-PLA + PEI-EVs + hGMSCs showed no cytotoxicity. However, 3D-PLA + PEI-EVs + hGMSCs exhibited greater osteogenic inductivity as revealed by morphological evaluation and transcriptomic analysis performed by next-generation sequencing (NGS). In addition, in vivo results showed that 3D-PLA + PEI-EVs + hGMSCs and 3D-PLA + PEI-EVs scaffolds implanted in rats subjected to cortical calvaria bone tissue damage were able to improve bone healing by showing better osteogenic properties. These results were supported also by computed tomography evaluation that revealed the repair of bone calvaria damage.
Conclusion: The re-establishing of the integrity of the bone lesions could be a promising strategy in the treatment of accidental or surgery trauma, especially for cranial bones.
Keywords: 3D scaffold; Bone regeneration; Extracellular vesicles; Human gingival mesenchymal stem cells.
Conflict of interest statement
Authors’ information
Not applicable.
Ethics approval and consent to participate
All experimental procedures were approved by the Ethics Committee of the University “G. d’Annunzio”, Chieti-Pescara and Ministry of Health (Italy). Written informed consent was obtained from all donors. All animal care and use was performed according to the European Organization Guidelines for Animal Welfare. The study has been authorized by the Ministry of Health “General Direction of animal health and veterinary drug” (Authorization 768/2016-PR 28/07/2016- D.lgs 26/2014). The experiments were planned in such a way as to minimize the total number of rats needed for the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Fig. 1
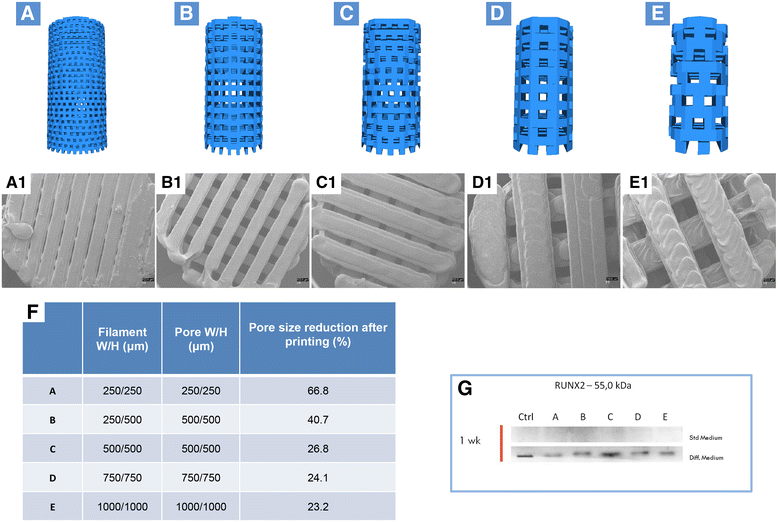
Design of standardized three-dimensional modular scaffolds (3D PLA) with different porosity and filament dimension. a Filament width/height 250/250 μm and pore width/height 250/250 μm; b Filament width/height 250/500 μm and pore width/height 250/500 μm; c Filament width/height 500/500 μm and pore width/height 500/500 μm; d Filament width/height 750/750 μm and pore width/height 750/750 μm; e Filament width/height 1000/1000 μm and pore width/height 1000/1000 μm. a1–e1 SEM micrographs of samples from a–e. Pore size reduction is present in each group. In-filament layering is observed especially in samples b–e. f Table with filament, porosity, and pore size reduction specifications. g RUNX2 expression in hGMSCs cultured in control and osteogenic medium. An increased expression of RUNX2 was present in cells cultured with scaffold design C (c)
Fig. 2
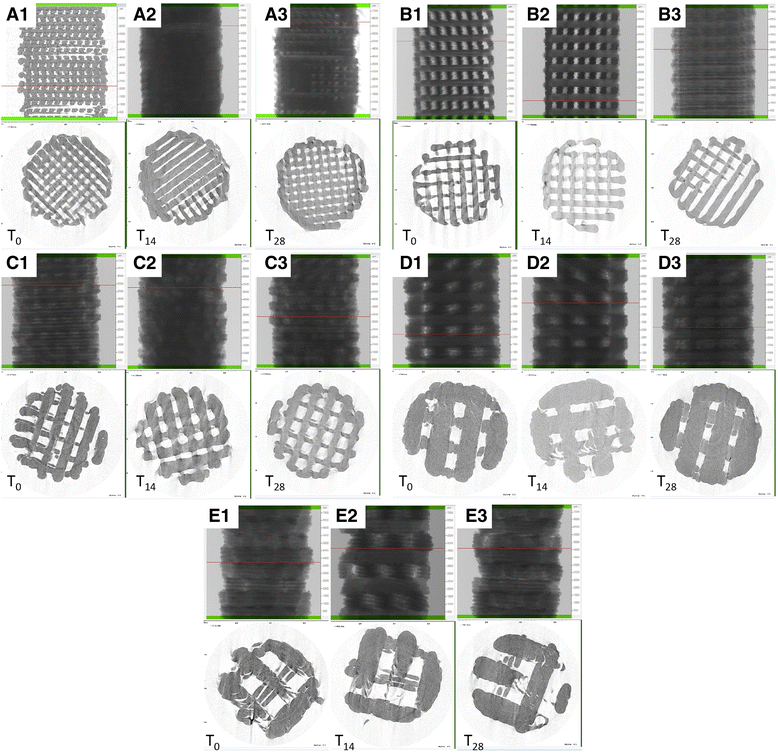
Microcomputed tomography three-dimensional rendering and evaluation of porosity, pore size, and wall thickness at different degradation time point. Scaffolds with a design A, b design B, c design C, d design D, and e design E at T0, T14, and T28. mCT values at T0 for porosity, trabecular thickness (TT), and trabecular separation (TS)
Fig. 3
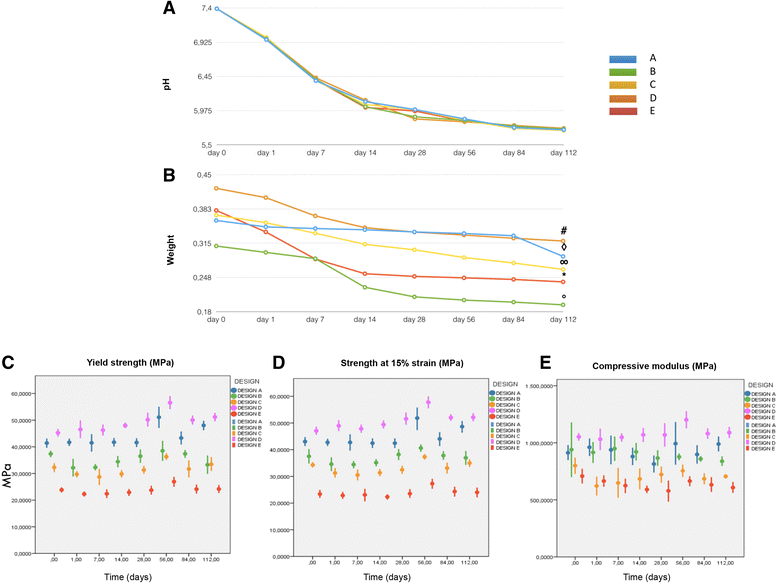
a Graph showing pH decrease during 3D-PLA degradation at different time points. b Graph showing weight loss during 3D PLA degradation at different time points. c Yield strength with significantly higher values for designs D over B, C, and E at day 0 and after 112 days. Design B showed a significant decrease over time. d Mean strength at 15% strain (±SD) for the tested groups for each time point. 3D PLA design D values were statistically higher than B, C, and E; differences between groups were confirmed after degradation except for design A which showed higher stability over time. e Compressive modulus with significantly higher values for design D over designs C and E at day 0 and after 112 days. Design B showed a significant decrease over time
Fig. 4
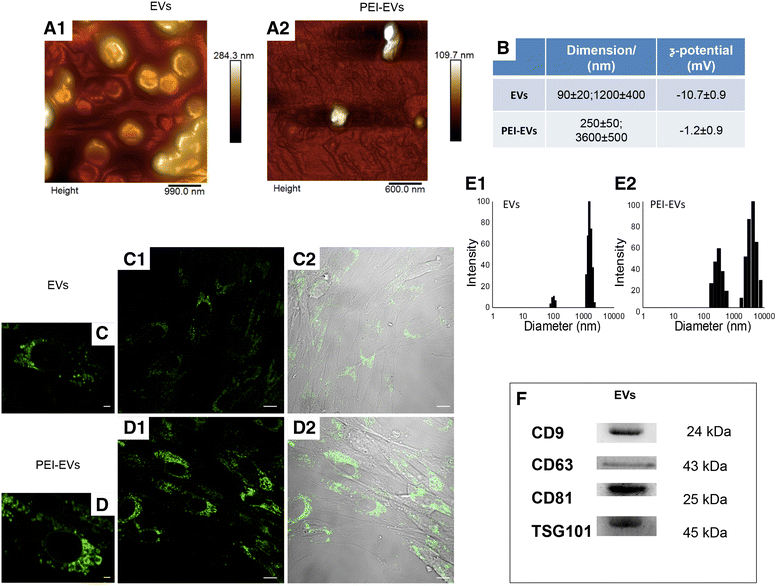
Extracellular vesicles (EVs) and polyethyleneimine (PEI)-engineered EVs (PEI-EVs) characterization. a Tapping mode topographic AFM image showing round morphology of EVs and PEI-EVs. b Average size and ζ-potential of EVs and PEI-EVs. High-magnification images (40×) of hGMSCs cured with c EVs and d PEI-EVs panel 1: hGMSCs cultured in the presence of WGA-stained EVs and PEI-EVs observed with confocal laser scanning microscopy after 24 h; panel 2: bright field of hGMSCs/WGA-stained EVs and PEI-EVs. e DLS size distribution histograms of EVs and PEI-EVs. f Western blot showed the positivity for CD9, CD63, CD81, and TSG101. Scale bars in c,d = 10 μm
Fig. 5
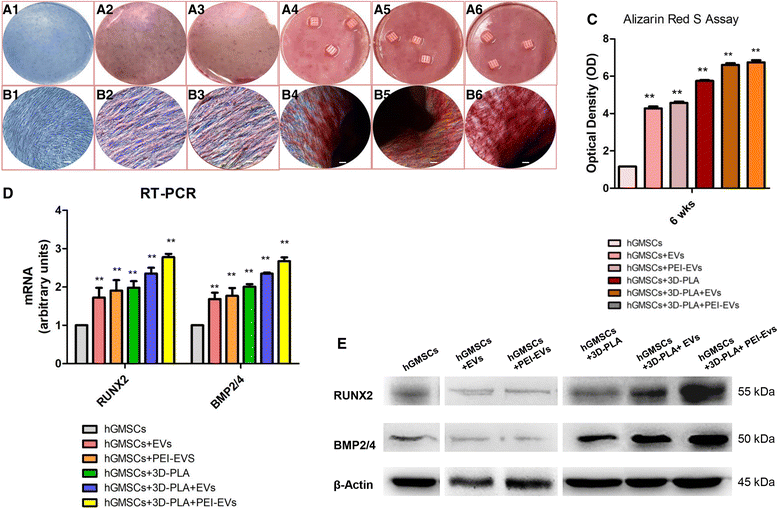
In vitro osteogenic induction. Human gingival mesenchymal stem cells (hGMSCs), maintained under osteogenic conditions for 6 weeks, were stained using Alizarin Red S solution. a Macro photographs. b Photographs obtained at light microscopy. Scale bars = 5 μm. c Graph optical density. d RT-PCR of osteogenic related markers. e Western blots of RUNX2 and BMP2/4; β-Actin was used as the housekeeping protein. **p < 0.01, versus hGMSCs. EVs, extracellular vesicles; PEI, polyethyleneimine
Fig. 6
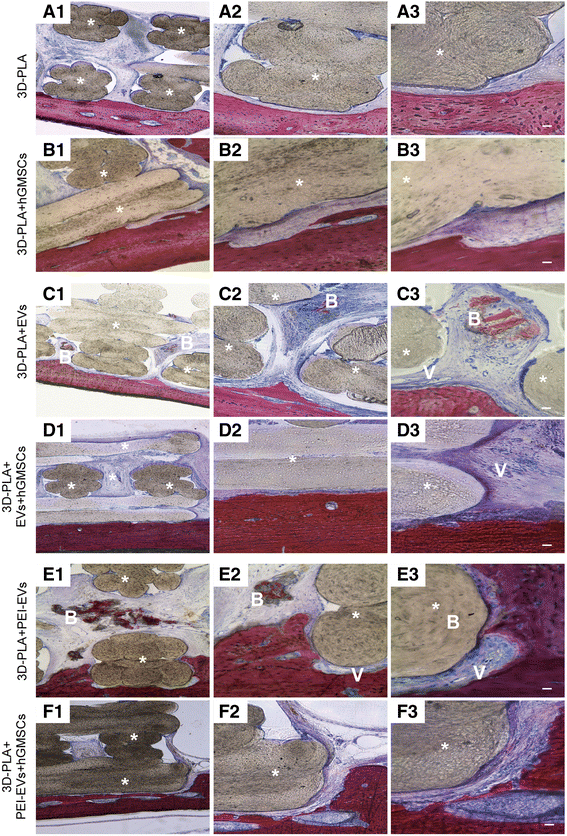
Histological evaluation. Samples harvested at 6 weeks after the calvarial defects were transplanted with a 3D-PLA scaffold or b 3D-PLA + human gingival mesenchymal stem cells (hGMSCs). Left panels (1): The images at low magnification (4×) showed 3D-PLA and 3D-PLA + hGMSCs integrated smoothly with the host tissue. Middle panels (2): High-magnification images (10×) showing the contact area between 3D-PLA and 3D-PLA + hGMSCs with bone calvaria grafted at 6 weeks postsurgery. Right panels (3): Images obtained at 40× objective showing the connective tissue between 3D-PLA and 3D-PLA + hGMSCs and bone host tissue. Samples harvested at 6 weeks after the calvarial defects was transplanted with c 3D-PLA + extracellular vesicles (EVs) scaffold or d 3D-PLA + EVs + hGMSCs. Left panels (1): The images at low magnification (4×) showed 3D-PLA + EVs and 3D-PLA + EVs + hGMSCs integrated smoothly with the host. Middle panels (2): High-magnification images (10×) showing the new bone formation stained with acid fuchsin in both samples grafted at 6 weeks postsurgery. Right panels (3): Images obtained at 40× objective showed a zone with new mineralized matrix inside 3D PLA scaffold for both samples. In particular, in the 3D-PLA + EVs (c3) sample in the contact zone some blood vessels are valuable. Samples harvested at 6 weeks after the calvarial defects was transplanted with e 3D-PLA + polyethyleneimine (PEI)-EVs scaffold or f 3D-PLA + PEI-EVs + hGMSCs. Left panels (1): The images at low magnification (4×) showed 3D-PLA + PEI-EVs and 3D-PLA + PEI-EVs + hGMSCs integrated smoothly with the host. Middle panels (2): High-magnification images (10×) showing the new bone formation stained with acid fuchsin in both samples grafted at 6 weeks postsurgery. Right panels (3): Images obtained at 40× objective showed new bone formation inside the scaffold structure. In particular, in 3D-PLA + PEI-EVs there were numerous blood vessels present around the new bone deposition area. Scale bars = 10 μm. *, scaffold; V, vessels; B, new bone. Acid fuchsin-toluidine blue staining
Fig. 7
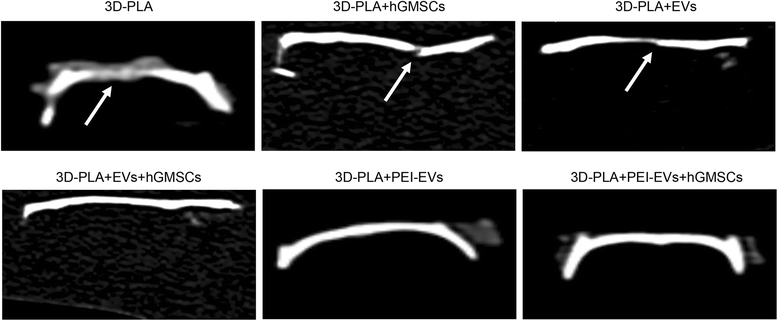
CT analysis showing bone damage was still present in the 3D-PLA, 3D-PLA + human gingival mesenchymal stem cells (hGMSCs), and 3D-PLA + extracellular vesicles (EVs) groups. On the contrary, in the 3D-PLA + EVs + hGMSCs, 3D-PLA + polyethyleneimine (PEI)-EVs, and 3D-PLA + PEI-EVs + hGMSCs groups the complete repair of the calvarial defect was shown. Arrows indicated bone defects
Fig. 8
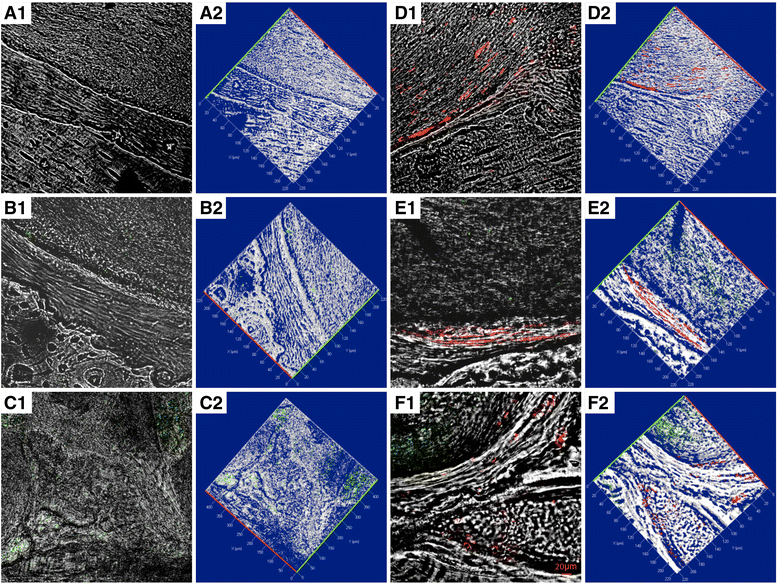
Confocal laser scanning microscopy analysis. a Confocal contrast image (left panel 1) of 3D-PLA (gray) grafted in mice calvaria after 6 weeks of implantation, with the corresponding 3D reconstruction image (right panel 2). b 3D-PLA (gray) and PKH-67-stained EVs (green) after 6 weeks of in vivo implantation, with the corresponding 3D reconstruction image. c 3D-PLA (gray) and PKH-67-stained PEI-EVs (green), with the corresponding 3D reconstruction image. d 3D-PLA (gray) and PKH-26-stained hGMSCs (red), with the corresponding 3D reconstruction image. e 3D-PLA (gray), PKH-26-stained hGMSCs (red), and PKH-67-stained EVs, with the corresponding 3D reconstruction image. f 3D-PLA (gray), PKH-26-stained hGMSCs (red), and PKH-67-stained PEI-EVs, with the corresponding 3D reconstruction image. Scale bars = 20 μm
Fig. 9
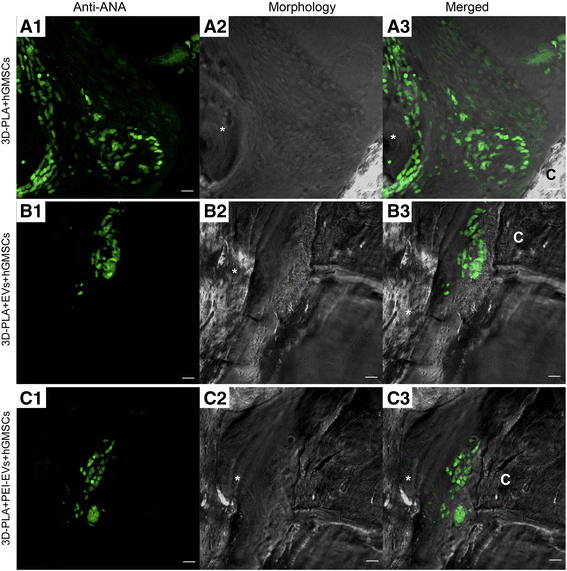
Confocal laser scanning microscopy analysis. Left panels: Human gingival mesenchymal stem cells (hGMSCs) anti-ANA positive nuclei in a 3D-PLA + hGMSCs, b 3D-PLA + extracellular vesicles (EVs) + hGMSCs, and c 3D-PLA + polyethyleneimine (PEI)-EVs + hGMSCs (green fluorescent). Middle panels: Confocal contrast image of 3D-PLA (gray) grafted in mice calvaria after 6 weeks of implantation. Right panels: Merged images of the two abovementioned channels. Scale bars = 10 μm. *, scaffold; C, rat calvaria
Similar articles
- Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration.
Diomede F, Gugliandolo A, Scionti D, Merciaro I, Cavalcanti MF, Mazzon E, Trubiani O. Diomede F, et al. Int J Mol Sci. 2018 Jan 23;19(2):329. doi: 10.3390/ijms19020329. Int J Mol Sci. 2018. PMID: 29360771 Free PMC article. - 3D Printing PLA/Gingival Stem Cells/ EVs Upregulate miR-2861 and -210 during Osteoangiogenesis Commitment.
Pizzicannella J, Diomede F, Gugliandolo A, Chiricosta L, Bramanti P, Merciaro I, Orsini T, Mazzon E, Trubiani O. Pizzicannella J, et al. Int J Mol Sci. 2019 Jul 2;20(13):3256. doi: 10.3390/ijms20133256. Int J Mol Sci. 2019. PMID: 31269731 Free PMC article. - Extracellular Vesicles Derived from Human Gingival Mesenchymal Stem Cells: A Transcriptomic Analysis.
Silvestro S, Chiricosta L, Gugliandolo A, Pizzicannella J, Diomede F, Bramanti P, Trubiani O, Mazzon E. Silvestro S, et al. Genes (Basel). 2020 Jan 21;11(2):118. doi: 10.3390/genes11020118. Genes (Basel). 2020. PMID: 31973135 Free PMC article. - Recent Advances in Endocrine, Metabolic and Immune Disorders: Mesenchymal Stem Cells (MSCs) and Engineered Scaffolds.
Cantore S, Crincoli V, Boccaccio A, Uva AE, Fiorentino M, Monno G, Bollero P, Derla C, Fabiano F, Ballini A, Santacroce L. Cantore S, et al. Endocr Metab Immune Disord Drug Targets. 2018;18(5):466-469. doi: 10.2174/1871530318666180423102905. Endocr Metab Immune Disord Drug Targets. 2018. PMID: 29692270 Review. - Extracellular vesicle and mesenchymal stem cells in bone regeneration: recent progress and perspectives.
Chu C, Wei S, Wang Y, Wang Y, Man Y, Qu Y. Chu C, et al. J Biomed Mater Res A. 2019 Jan;107(1):243-250. doi: 10.1002/jbm.a.36518. Epub 2018 Oct 31. J Biomed Mater Res A. 2019. PMID: 30378760 Review.
Cited by
- The Delivery of Extracellular Vesicles Loaded in Biomaterial Scaffolds for Bone Regeneration.
Yan HC, Yu TT, Li J, Qiao YQ, Wang LC, Zhang T, Li Q, Zhou YH, Liu DW. Yan HC, et al. Front Bioeng Biotechnol. 2020 Aug 19;8:1015. doi: 10.3389/fbioe.2020.01015. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32974327 Free PMC article. Review. - Emergence of the Stem Cell Secretome in Regenerative Engineering.
Daneshmandi L, Shah S, Jafari T, Bhattacharjee M, Momah D, Saveh-Shemshaki N, Lo KW, Laurencin CT. Daneshmandi L, et al. Trends Biotechnol. 2020 Dec;38(12):1373-1384. doi: 10.1016/j.tibtech.2020.04.013. Epub 2020 Jul 1. Trends Biotechnol. 2020. PMID: 32622558 Free PMC article. Review. - Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration.
Wang X, Thomsen P. Wang X, et al. Basic Clin Pharmacol Toxicol. 2021 Jan;128(1):18-36. doi: 10.1111/bcpt.13478. Epub 2020 Sep 22. Basic Clin Pharmacol Toxicol. 2021. PMID: 32780530 Free PMC article. Review. - Mesenchymal stem cell-derived extracellular vesicles: a possible therapeutic strategy for orthopaedic diseases: a narrative review.
Zeng ZL, Xie H. Zeng ZL, et al. Biomater Transl. 2022 Sep 28;3(3):175-187. doi: 10.12336/biomatertransl.2022.03.002. eCollection 2022. Biomater Transl. 2022. PMID: 36654775 Free PMC article. Review. - Bovine pericardium membrane, gingival stem cells, and ascorbic acid: a novel team in regenerative medicine.
Pizzicannella J, Marconi GD, Pierdomenico SD, Cavalcanti MFXB, Diomede F, Trubiani O. Pizzicannella J, et al. Eur J Histochem. 2019 Sep 25;63(3):3064. doi: 10.4081/ejh.2019.3064. Eur J Histochem. 2019. PMID: 31696691 Free PMC article.
References
- Szpalski C, Barr J, Wetterau M, Saadeh PB, Warren SM. Cranial bone defects: current and future strategies. Neurosurg Focus. 2010;29(6):E8. 10.3171/2010.9.FOCUS10201. - PubMed
- Bose S, Vahabzadeh S, Bandyopadhyay A. Bone tissue engineering using 3D printing. Mater Today. 2013;16(12):496–504. doi: 10.1016/j.mattod.2013.11.017. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous