Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden - PubMed (original) (raw)
Clinical Trial
. 2018 May 31;378(22):2093-2104.
doi: 10.1056/NEJMoa1801946. Epub 2018 Apr 16.
Tudor-Eliade Ciuleanu 1, Adam Pluzanski 1, Jong Seok Lee 1, Gregory A Otterson 1, Clarisse Audigier-Valette 1, Elisa Minenza 1, Helena Linardou 1, Sjaak Burgers 1, Pamela Salman 1, Hossein Borghaei 1, Suresh S Ramalingam 1, Julie Brahmer 1, Martin Reck 1, Kenneth J O'Byrne 1, William J Geese 1, George Green 1, Han Chang 1, Joseph Szustakowski 1, Prabhu Bhagavatheeswaran 1, Diane Healey 1, Yali Fu 1, Faith Nathan 1, Luis Paz-Ares 1
Affiliations
- PMID: 29658845
- PMCID: PMC7193684
- DOI: 10.1056/NEJMoa1801946
Clinical Trial
Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden
Matthew D Hellmann et al. N Engl J Med. 2018.
Abstract
Background: Nivolumab plus ipilimumab showed promising efficacy for the treatment of non-small-cell lung cancer (NSCLC) in a phase 1 trial, and tumor mutational burden has emerged as a potential biomarker of benefit. In this part of an open-label, multipart, phase 3 trial, we examined progression-free survival with nivolumab plus ipilimumab versus chemotherapy among patients with a high tumor mutational burden (≥10 mutations per megabase).
Methods: We enrolled patients with stage IV or recurrent NSCLC that was not previously treated with chemotherapy. Those with a level of tumor programmed death ligand 1 (PD-L1) expression of at least 1% were randomly assigned, in a 1:1:1 ratio, to receive nivolumab plus ipilimumab, nivolumab monotherapy, or chemotherapy; those with a tumor PD-L1 expression level of less than 1% were randomly assigned, in a 1:1:1 ratio, to receive nivolumab plus ipilimumab, nivolumab plus chemotherapy, or chemotherapy. Tumor mutational burden was determined by the FoundationOne CDx assay.
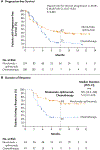
Results: Progression-free survival among patients with a high tumor mutational burden was significantly longer with nivolumab plus ipilimumab than with chemotherapy. The 1-year progression-free survival rate was 42.6% with nivolumab plus ipilimumab versus 13.2% with chemotherapy, and the median progression-free survival was 7.2 months (95% confidence interval [CI], 5.5 to 13.2) versus 5.5 months (95% CI, 4.4 to 5.8) (hazard ratio for disease progression or death, 0.58; 97.5% CI, 0.41 to 0.81; P<0.001). The objective response rate was 45.3% with nivolumab plus ipilimumab and 26.9% with chemotherapy. The benefit of nivolumab plus ipilimumab over chemotherapy was broadly consistent within subgroups, including patients with a PD-L1 expression level of at least 1% and those with a level of less than 1%. The rate of grade 3 or 4 treatment-related adverse events was 31.2% with nivolumab plus ipilimumab and 36.1% with chemotherapy. ical; CheckMate 227 ClinicalTrials.gov number, NCT02477826 .).
Conclusions: Progression-free survival was significantly longer with first-line nivolumab plus ipilimumab than with chemotherapy among patients with NSCLC and a high tumor mutational burden, irrespective of PD-L1 expression level. The results validate the benefit of nivolumab plus ipilimumab in NSCLC and the role of tumor mutational burden as a biomarker for patient selection. (Funded by Bristol-Myers Squibb and Ono Pharmaceut
Figures
Figure 1.. Trial Design.
Chemotherapy for patients with nonsquamous non–small-cell lung cancer (NSCLC) consisted of pemetrexed (500 mg per square meter of body-surface area) plus cisplatin (75 mg per square meter) or carboplatin (area under the concentration-time curve [AUC], 5 or 6), every 3 weeks for up to four cycles, with optional maintenance therapy with pemetrexed (500 mg per square meter) after chemotherapy or with nivolumab (360 mg every 3 weeks) plus pemetrexed (500 mg per square meter) after nivolumab plus chemotherapy. Chemotherapy for patients with squamous NSCLC consisted of gemcitabine (1000 or 1250 mg per square meter) plus cisplatin (75 mg per square meter), or gemcitabine (1000 mg per square meter) plus carboplatin (AUC, 5), every 3 weeks for up to four cycles. The tumor mutational burden (TMB) coprimary analysis was conducted in the subgroup of patients assigned to nivolumab plus ipilimumab or chemotherapy who had a TMB of at least 10 mutations per megabase. Given the recommendation of the data and safety monitoring committee to continue the trial for overall survival, analysis of the coprimary end point of overall survival among patients selected on the basis of the programmed death ligand 1 (PD-L1) expression level was not performed for the current database lock. Eastern Cooperative Oncology Group (ECOG) performance-status scores range from 0 to 5, with higher scores indicating greater disability.
Figure 2.. Efficacy of Nivolumab plus Ipilimumab versus Chemotherapy in Patients with a High Tumor Mutational Burden.
A high tumor mutational burden was defined as at least 10 mutations per megabase. In Panel A, the 95% confidence interval for the hazard ratio for disease progression or death was 0.43 to 0.77. In both panels, the circles (for nivolumab plus ipilimumab) and triangles (for chemotherapy) indicate censored data. NR denotes not reached.
Figure 3.. Progression-free Survival among Patients with a High Tumor Mutational Burden According to Tumor PD-L1 Expression and Histologic Type.
A high tumor mutational burden was defined as at least 10 mutations per megabase. The circles (for nivolumab plus ipilimumab) and triangles (for chemotherapy) indicate censored data.
Figure 4.. Subgroup Analyses of Progression-free Survival among Patients with a High Tumor Mutational Burden.
A high tumor mutational burden was defined as at least 10 mutations per megabase. The subgroup of patients who had never smoked could not be evaluated owing to the small sample size.
Comment in
- High TMB Predicts Immunotherapy Benefit.
[No authors listed] [No authors listed] Cancer Discov. 2018 Jun;8(6):668. doi: 10.1158/2159-8290.CD-NB2018-048. Epub 2018 Apr 16. Cancer Discov. 2018. PMID: 29661758 - Nivolumab-ipilimumab - exploiting the mutation burden of NSCLCs.
Killock D. Killock D. Nat Rev Clin Oncol. 2018 Jul;15(7):403. doi: 10.1038/s41571-018-0034-y. Nat Rev Clin Oncol. 2018. PMID: 29703915 No abstract available. - New windows open for immunotherapy in lung cancer.
Hendriks L, Besse B. Hendriks L, et al. Nature. 2018 Jun;558(7710):376-377. doi: 10.1038/d41586-018-05312-9. Nature. 2018. PMID: 29907821 No abstract available. - Immune Checkpoint Blockade across the Cancer Care Continuum.
Helmink BA, Gaudreau PO, Wargo JA. Helmink BA, et al. Immunity. 2018 Jun 19;48(6):1077-1080. doi: 10.1016/j.immuni.2018.06.003. Immunity. 2018. PMID: 29924973 - Frontline immunotherapy for NSCLC: alone or not alone?
Gridelli C, Casaluce F. Gridelli C, et al. Nat Rev Clin Oncol. 2018 Oct;15(10):593-594. doi: 10.1038/s41571-018-0070-7. Nat Rev Clin Oncol. 2018. PMID: 29993034 No abstract available. - Radiotherapy, tumor mutational burden, and immune checkpoint inhibitors: time to do the math.
Giordano FA, Veldwijk MR, Herskind C, Wenz F. Giordano FA, et al. Strahlenther Onkol. 2018 Oct;194(10):873-875. doi: 10.1007/s00066-018-1341-z. Epub 2018 Jul 20. Strahlenther Onkol. 2018. PMID: 30030581 No abstract available. - Predictive biomarkers for immune checkpoint inhibitor therapy: we need to keep searching.
Huerter MM, Ganti AK. Huerter MM, et al. J Thorac Dis. 2018 Jul;10(Suppl 18):S2195-S2197. doi: 10.21037/jtd.2018.06.144. J Thorac Dis. 2018. PMID: 30123559 Free PMC article. No abstract available. - Lung Cancer with a High Tumor Mutational Burden.
VanderLaan PA, Rangachari D, Costa DB. VanderLaan PA, et al. N Engl J Med. 2018 Sep 13;379(11):1093. doi: 10.1056/NEJMc1808566. N Engl J Med. 2018. PMID: 30211492 No abstract available. - Tumor mutation burden in lung cancer: a new predictive biomarker for immunotherapy or too soon to tell?
Alexander M, Galeas J, Cheng H. Alexander M, et al. J Thorac Dis. 2018 Nov;10(Suppl 33):S3994-S3998. doi: 10.21037/jtd.2018.09.35. J Thorac Dis. 2018. PMID: 30631537 Free PMC article. No abstract available. - Immunotherapy in Non-Small Cell Lung Cancer. Which Patients and at Which Stage?
Allinson JP, Brown J, Gibb K, Navani N. Allinson JP, et al. Am J Respir Crit Care Med. 2019 May 15;199(10):1277-1279. doi: 10.1164/rccm.201810-1930RR. Am J Respir Crit Care Med. 2019. PMID: 30860861 No abstract available.
Similar articles
- Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer.
Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora Jimenez E, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O'Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS. Hellmann MD, et al. N Engl J Med. 2019 Nov 21;381(21):2020-2031. doi: 10.1056/NEJMoa1910231. Epub 2019 Sep 28. N Engl J Med. 2019. PMID: 31562796 Clinical Trial. - First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers.
Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, Borghaei H, Jolivet J, Horn L, Mates M, Brahmer J, Rabinowitz I, Reddy PS, Chesney J, Orcutt J, Spigel DR, Reck M, O'Byrne KJ, Paz-Ares L, Hu W, Zerba K, Li X, Lestini B, Geese WJ, Szustakowski JD, Green G, Chang H, Ramalingam SS. Ready N, et al. J Clin Oncol. 2019 Apr 20;37(12):992-1000. doi: 10.1200/JCO.18.01042. Epub 2019 Feb 20. J Clin Oncol. 2019. PMID: 30785829 Free PMC article. Clinical Trial. - Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma.
Doki Y, Ajani JA, Kato K, Xu J, Wyrwicz L, Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, El Hajbi F, Di Bartolomeo M, Braghiroli MI, Holtved E, Ostoich SA, Kim HR, Ueno M, Mansoor W, Yang WC, Liu T, Bridgewater J, Makino T, Xynos I, Liu X, Lei M, Kondo K, Patel A, Gricar J, Chau I, Kitagawa Y; CheckMate 648 Trial Investigators. Doki Y, et al. N Engl J Med. 2022 Feb 3;386(5):449-462. doi: 10.1056/NEJMoa2111380. N Engl J Med. 2022. PMID: 35108470 Clinical Trial. - Nivolumab plus ipilimumab in non-small-cell lung cancer.
Reck M, Borghaei H, O'Byrne KJ. Reck M, et al. Future Oncol. 2019 Jul;15(19):2287-2302. doi: 10.2217/fon-2019-0031. Epub 2019 May 8. Future Oncol. 2019. PMID: 31066582 Review. - Anti-PD-1/PD-L1 antibody therapy for pretreated advanced nonsmall-cell lung cancer: A meta-analysis of randomized clinical trials.
Zhou GW, Xiong Y, Chen S, Xia F, Li Q, Hu J. Zhou GW, et al. Medicine (Baltimore). 2016 Aug;95(35):e4611. doi: 10.1097/MD.0000000000004611. Medicine (Baltimore). 2016. PMID: 27583876 Free PMC article. Review.
Cited by
- Prognostic significance of the modified Glasgow Prognostic Score in NSCLC patients undergoing immune checkpoint inhibitor therapy: a meta-analysis.
Wu J, Wang H, Yang R, Liu D, Li W. Wu J, et al. Front Oncol. 2024 Oct 11;14:1449853. doi: 10.3389/fonc.2024.1449853. eCollection 2024. Front Oncol. 2024. PMID: 39464717 Free PMC article. - [Predictive diagnostics for checkpoint inhibitors].
Schildhaus HU, Weichert W. Schildhaus HU, et al. Pathologe. 2021 Jul;42(4):380-390. doi: 10.1007/s00292-021-00939-4. Epub 2021 May 6. Pathologe. 2021. PMID: 33956171 Review. German. - A novel panel based on immune infiltration and tumor mutational burden for prognostic prediction in hepatocellular carcinoma.
Xie C, Wu H, Pan T, Zheng X, Yang X, Zhang G, Lian Y, Lin J, Peng L. Xie C, et al. Aging (Albany NY). 2021 Mar 10;13(6):8563-8587. doi: 10.18632/aging.202670. Epub 2021 Mar 10. Aging (Albany NY). 2021. PMID: 33714200 Free PMC article. - The development of a novel signature based on the m6A RNA methylation regulator-related ceRNA network to predict prognosis and therapy response in sarcomas.
Li H, Lin D, Wang X, Feng Z, Zhang J, Wang K. Li H, et al. Front Genet. 2022 Oct 12;13:894080. doi: 10.3389/fgene.2022.894080. eCollection 2022. Front Genet. 2022. PMID: 36313417 Free PMC article. - Based on different immune responses under the glucose metabolizing type of papillary thyroid cancer and the response to anti-PD-1 therapy.
Xie W, Zeng Y, Hu L, Hao J, Chen Y, Yun X, Lin Q, Li H. Xie W, et al. Front Immunol. 2022 Sep 8;13:991656. doi: 10.3389/fimmu.2022.991656. eCollection 2022. Front Immunol. 2022. PMID: 36211409 Free PMC article.
References
- Hanna N, Johnson D, Temin S, Masters G. Systemic therapy for stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update summary. J Oncol Pract 2017; 13: 832–7. - PubMed
- Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017; 15: 504–35. - PubMed
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med 2016; 375: 1823–33. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials