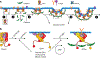Regulation of Clathrin-Mediated Endocytosis - PubMed (original) (raw)
Review
Regulation of Clathrin-Mediated Endocytosis
Marcel Mettlen et al. Annu Rev Biochem. 2018.
Abstract
Clathrin-mediated endocytosis (CME) is the major endocytic pathway in mammalian cells. It is responsible for the uptake of transmembrane receptors and transporters, for remodeling plasma membrane composition in response to environmental changes, and for regulating cell surface signaling. CME occurs via the assembly and maturation of clathrin-coated pits that concentrate cargo as they invaginate and pinch off to form clathrin-coated vesicles. In addition to the major coat proteins, clathrin triskelia and adaptor protein complexes, CME requires a myriad of endocytic accessory proteins and phosphatidylinositol lipids. CME is regulated at multiple steps-initiation, cargo selection, maturation, and fission-and is monitored by an endocytic checkpoint that induces disassembly of defective pits. Regulation occurs via posttranslational modifications, allosteric conformational changes, and isoform and splice-variant differences among components of the CME machinery, including the GTPase dynamin. This review summarizes recent findings on the regulation of CME and the evolution of this complex process.
Keywords: AP2; adaptor protein-2; dynamin; endocytic accessory proteins; endocytic checkpoint; evolution; signaling.
Figures
Figure 1
Clathrin-mediated endocytosis is regulated by multiple factors at multiple stages. Clathrin-mediated endocytosis is a multistage process involving the initiation and stabilization of nascent CCPs, maturation and curvature generation, and finally dynamin-catalyzed fission. Each step is regulated by multiple inputs. Abbreviations: AAK1, adaptor associated kinase 1; AP2, adaptor protein-2; EAPs, endocytic accessory proteins; PIP, phosphatidylinositol phosphate. Figure modified from Schmid (107).
Figure 2
The central role of AP2 in regulating CCP initiation and stabilization. (a) The allosteric regulation of AP2 conformational changes controls early steps in CME, including: (❶) the recruitment of cytosolic AP2 in the closed conformation to the PM, where (❷) PI4,5P2 binding releases the β2 hinge to nucleate clathrin assembly; (❸) cargo and PI4,5P2 binding to the μ2 subunit, as well as interactions with EAPs, trigger the open conformation to stabilize the growing, nascent CCP and prevent early abortive events; (❹) sustained PI4,5P2 interactions with α, β2, and μ2 binding sites, as well as clathrin-stimulated AAK1 phosphorylation of μ2, supports further cargo recruitment, CCP growth, and (❺) maturation. Boxes correspond to sequential AP2 conformational changes enlarged in panel b. (b) Sequential interactions between PI4,5P2 and α, β2, and μ2 subunits, as well as with pioneer EAPs, trigger and stabilize conformational changes that activate AP2. AAK1, which phosphorylates μ2 to stabilize the open conformation, is activated by assembled clathrin, thus providing a positive feedback loop during CCP maturation. Abbreviations: AAK1, adaptor associated kinase 1; AP2, adaptor protein-2; CALM, clathrin assembly lymphoid myeloid leukemia; CCP, clathrin-coated pit; CME, clathrin-mediated endocytosis; EAPs, endocytic accessory proteins; Eps15, EGF-receptor substrate 15; FCHo, Fer/Cip4 homology domain-only protein; NECAP, adaptin-ear-binding coat-associated protein; PI4,5P2, phosphatidylinositol lipid; PM, plasma membrane.
Figure 3
The endocytic checkpoint hypothesis. A hypothetical endocytic checkpoint monitors the fidelity of CCP maturation. Deficiencies in several endocytic accessory proteins lead to decreased efficiency of CCP maturation and increased rates of turnover of abortive CCPs. Small interfering RNA-mediated knockdown of dynamin-2 decreases the rate of abortive CCP turnover. Based on these data, we speculate that the properties of nascent CCPs, such as coat assembly, curvature generation, and cargo concentration, are monitored by SH3 domain-containing dynamin binding partners that also interact with coat components, cargo, and/or sense curvature and kinetically control dynamin assembly. Together, these interactions function to monitor the fidelity of CCP maturation. Much remains to be done to test this hypothesis and to identify components of the endocytic checkpoint apparatus and the mechanisms to sense and turnover aberrant CCP intermediates. Abbreviations: AD, appendage domain; AP2, adaptor protein-2; CALM, clathrin assembly lymphoid myeloid leukemia; CCP, clathrin-coated pit; CCV, clathrin-coated vesicle; CLCs, clathrin light chains; SH3, Src-homology domain 3; TfnR, transferrin receptor. Figure modified from Schmid (107).
Figure 4
Regulation of dynamin by posttranslational modifications, intramolecular interactions, and splice variants. The GTPase dynamin consists of five functional domains and exists as a tetramer in solution (PDB ID 53AF; 152). Dynamin is regulated by: (a) phosphorylation of the PRD, which modulates SH3 domain binding; (b) SH3 domain-containing partners that bind the PRD and can either stimulate or inhibit dynamin assembly and/or GTPase activity; (c) intramolecular interactions between the PHD and stalk that prevent higher order assembly; and (d) splice variants within the PRD that alter interactions with kinases or phosphatases or splice variants within the middle domain that affect allosteric conformational changes and modulate the effects of SH3 domain interactions on dynamin assembly. Dynamin is a key regulator of CME; therefore, regulation of dynamin can impact multiple stages of CME. Abbreviations: CME, clathrin-mediated endocytosis; GED, GTPase effector domain; PDB, Protein Data Bank; PHD, pleckstrin homology domain; PRD, proline/arginine-rich domain; SH3, Src-homology 3 domain.
Similar articles
- Regulation of clathrin-mediated endocytosis by hierarchical allosteric activation of AP2.
Kadlecova Z, Spielman SJ, Loerke D, Mohanakrishnan A, Reed DK, Schmid SL. Kadlecova Z, et al. J Cell Biol. 2017 Jan 2;216(1):167-179. doi: 10.1083/jcb.201608071. Epub 2016 Dec 21. J Cell Biol. 2017. PMID: 28003333 Free PMC article. - Temporal Ordering in Endocytic Clathrin-Coated Vesicle Formation via AP2 Phosphorylation.
Wrobel AG, Kadlecova Z, Kamenicky J, Yang JC, Herrmann T, Kelly BT, McCoy AJ, Evans PR, Martin S, Müller S, Salomon S, Sroubek F, Neuhaus D, Höning S, Owen DJ. Wrobel AG, et al. Dev Cell. 2019 Aug 19;50(4):494-508.e11. doi: 10.1016/j.devcel.2019.07.017. Dev Cell. 2019. PMID: 31430451 Free PMC article. - Clathrin promotes incorporation of cargo into coated pits by activation of the AP2 adaptor micro2 kinase.
Jackson AP, Flett A, Smythe C, Hufton L, Wettey FR, Smythe E. Jackson AP, et al. J Cell Biol. 2003 Oct 27;163(2):231-6. doi: 10.1083/jcb.200304079. J Cell Biol. 2003. PMID: 14581451 Free PMC article. - Evolving models for assembling and shaping clathrin-coated pits.
Chen Z, Schmid SL. Chen Z, et al. J Cell Biol. 2020 Sep 7;219(9):e202005126. doi: 10.1083/jcb.202005126. J Cell Biol. 2020. PMID: 32770195 Free PMC article. Review. - Sorting it out: AP-2 and alternate clathrin adaptors in endocytic cargo selection.
Traub LM. Traub LM. J Cell Biol. 2003 Oct 27;163(2):203-8. doi: 10.1083/jcb.200309175. J Cell Biol. 2003. PMID: 14581447 Free PMC article. Review.
Cited by
- Phosphoinositide species and filamentous actin formation mediate engulfment by senescent tumor cells.
Frey WD, Anderson AY, Lee H, Nguyen JB, Cowles EL, Lu H, Jackson JG. Frey WD, et al. PLoS Biol. 2022 Oct 24;20(10):e3001858. doi: 10.1371/journal.pbio.3001858. eCollection 2022 Oct. PLoS Biol. 2022. PMID: 36279312 Free PMC article. - A conformational switch in clathrin light chain regulates lattice structure and endocytosis at the plasma membrane of mammalian cells.
Obashi K, Sochacki KA, Strub MP, Taraska JW. Obashi K, et al. Nat Commun. 2023 Feb 9;14(1):732. doi: 10.1038/s41467-023-36304-7. Nat Commun. 2023. PMID: 36759616 Free PMC article. - Endosomal Escape and Cytosolic Penetration of Macromolecules Mediated by Synthetic Delivery Agents.
Brock DJ, Kondow-McConaghy HM, Hager EC, Pellois JP. Brock DJ, et al. Bioconjug Chem. 2019 Feb 20;30(2):293-304. doi: 10.1021/acs.bioconjchem.8b00799. Epub 2018 Dec 6. Bioconjug Chem. 2019. PMID: 30462487 Free PMC article. Review. - The Effect of Sleep Deprivation and Subsequent Recovery Period on the Synaptic Proteome of Rat Cerebral Cortex.
Gulyássy P, Todorov-Völgyi K, Tóth V, Györffy BA, Puska G, Simor A, Juhász G, Drahos L, Kékesi KA. Gulyássy P, et al. Mol Neurobiol. 2022 Feb;59(2):1301-1319. doi: 10.1007/s12035-021-02699-x. Epub 2022 Jan 5. Mol Neurobiol. 2022. PMID: 34988919 Free PMC article. - Disruption of endocytosis through chemical inhibition of clathrin heavy chain function.
Dejonghe W, Sharma I, Denoo B, De Munck S, Lu Q, Mishev K, Bulut H, Mylle E, De Rycke R, Vasileva M, Savatin DV, Nerinckx W, Staes A, Drozdzecki A, Audenaert D, Yperman K, Madder A, Friml J, Van Damme D, Gevaert K, Haucke V, Savvides SN, Winne J, Russinova E. Dejonghe W, et al. Nat Chem Biol. 2019 Jun;15(6):641-649. doi: 10.1038/s41589-019-0262-1. Epub 2019 Apr 22. Nat Chem Biol. 2019. PMID: 31011214 Free PMC article.
References
- Robinson MS. 2015. Forty years of clathrin-coated vesicles. Traffic 16:1210–38 - PubMed
- Schmid EM, McMahon HT. 2007. Integrating molecular and network biology to decode endocytosis. Nature 448:883–88 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM042455/GM/NIGMS NIH HHS/United States
- R01 GM073165/GM/NIGMS NIH HHS/United States
- R01 MH061345/MH/NIMH NIH HHS/United States
- R37 MH061345/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials