Evolutionary convergence and divergence in archaeal chromosomal proteins and Chromo-like domains from bacteria and eukaryotes - PubMed (original) (raw)
Evolutionary convergence and divergence in archaeal chromosomal proteins and Chromo-like domains from bacteria and eukaryotes
Gurmeet Kaur et al. Sci Rep. 2018.
Abstract
SH3-fold-β-barrel domains of the chromo-like superfamily recognize epigenetic marks in eukaryotic proteins. Their provenance has been placed either in archaea, based on apparent structural similarity to chromatin-compacting Sul7d and Cren7 proteins, or in bacteria based on the presence of sequence homologs. Using sequence and structural evidence we establish that the archaeal Cren7/Sul7 proteins emerged from a zinc ribbon (ZnR) ancestor. Further, we show that the ancestral eukaryotic chromo-like domains evolved from bacterial versions, likely acquired from early endosymbioses, which already possessed an aromatic cage for recognition of modified amino-groups. These bacterial versions are part of a radiation of secreted SH3-fold domains, which spawned both chromo-like domains and classical SH3 domains in the context of peptide-recognition in the peptidoglycan or the extracellular matrix. This establishes that Cren7/Sul7 converged to a "SH3"-like state from a ZnR precursor via the loss of metal-chelation and acquisition of stronger hydrophobic interactions; it is unlikely to have participated in the evolution of the chromo-like domains. We show that archaea possess several Cren7/Sul7-related proteins with intact Zn-chelating ligands, which we predict to play previously unstudied roles in chromosome segregation during cell-division comparable to the PRC barrel and CdvA domain proteins.
Conflict of interest statement
The authors declare no competing interests.
Figures
Figure 1
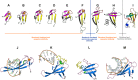
Comparative structural view of the major secondary elements (A–I) and binding interfaces (J–M) in ZnRs, SH3s and Cren7. (A) Type-1A ZnR (PR domain-containing protein 11, PDB: 3RAY_A) (B) Type-1B zinc ribbon (Rubrerythrin, PDB: 1NNQ_A) (C) Type-2 zinc ribbon (Archaeal exosome RNA binding protein CSL4, PDB: 2BA1_A) (D) Type-2 zinc ribbon (Uncharacterized BCR cg1592, PDB: 2JNY_A) (E) ZnRs with three strands on each side (Anaerobic ribonucleoside-triphosphate reductase, PDB: 1H8K_A) (F) Type-2 zinc finger (Transcriptional Elongation Factor SII, PDB: 1TFI_A) (G) Cren7 (PDB: 3KXT_A) (H) Sso7 (PDB: 1C8C_A) (I) SH3-like fold (Tudor domain-containing protein 3, PDB: 3PNW_O). (J) DNA bound zinc ribbon domain (putative integrase [Bacteriophage A118], PDB: 4KIS_A) (K) DNA and peptide bound chromodomain (Male specific Lethal-3, PDB: 3OA6_A) (L) peptide bound SH3 domain (Tyrosine-protein kinase ABL1, PDB: 1BBZ_A) (M) DNA bound Cren7 (PDB: 3KXT_A). In all the panels, the Zn ion is represented by an orange sphere, side chains of zinc-chelating and other functionally-important amino acids are represented as sticks, bound peptides are yellow colored stick, the sugar-phosphate backbone of DNA is orange with nucleotides in green. Color scheme for (A–I): For type-2 zinc ribbons, the N-terminal β-hairpin is colored purple, the third β-strand is colored gray, the zinc knuckles are colored red and the C-terminal β-hairpin is colored yellow. The equivalent β-strands in type-2, -1A and -1B are colored alike. The Tudor domain is colored in a gradient of blue to red from the N- to the C-terminal. In panels (J–M): All β-strands are colored in blue, all loops in pink and α-helices in green.
Figure 2
Multiple sequence alignment (MSA) and domain architectures of Cren7 proteins. (A) Structure-based MSA of representative sequences of Sul7, Cren7, Cren7Znr and bona fide ZnRs. Accession number or the PDB identifier, and the alignment range are indicated for each sequence. For Sul7, Cren7 and Cren7Znr proteins, the organism names are also mentioned after the accession number/PDB. Zn-chelating residues are boxed in black and small amino acids (Gly, Pro) just after the Zn-binding ligands are colored in red. At positions with a 70% consensus, charged or polar residues are highlighted in blue, and hydrophobic, apolar and aliphatic residues are in yellow. Some insertions are not displayed for clarity, and the number of omitted residues is indicated. Regions of circular permutation in ZnRs are separated by a blue ‘|’ mark and the sequence numbers of the permuted ZnRs are shown in red. The consensus secondary structures are depicted above the alignment. (B) Domain architectures of Cren7 and Cren7Znr proteins. Representative sequence identifier with organism name are mention below individual architectures.
Figure 3
Multiple sequence alignment (MSA), probable phylogeny and domain architectures of chromo-like domains. (A) MSA of representative bacterial chromo-like domains. (B) Structure-based MSA of representative eukaryotic chromo-like domains. Coloring scheme for panel (A) and (B) follows Fig. 2(A). The amino acids at positions that are likely to be involved in forming the peptide-binding aromatic cage are highlighted in orange and marked with the asterisk above the MSA. (C) View of the peptide-binding aromatic cage of Arabidopsis Morf Related Gene (MRG) group protein MRG2 chromo domain (PDB: 4PL6). The aromatic cage is marked by a dashed ellipse and the aromatic residue positions from the N- to C- terminus are marked by numbers 1–5. (D) Probable phylogenetic relationships between the various chromo-like domains. (E) Domain architectures of bacterial chromo domains. Parallel architectures among bacterial chromo and bacterial SH3 domains are grouped at the bottom. SP indicates signal peptide. Besides the architectures shown in the figure, there are various other bacteria proteins with 2 to 14 tandem SH3 domains and 2 to 6 tandem chromo domains. (see Supplementary Material S3 for details).
Figure 4
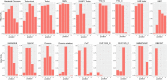
Comparative graphical view of the conservation of aromatic residues at the five positions that make up the peptide-binding aromatic cage in chromo-like domains. Individual graphs are labelled on the top by the family they represent. The X-axis represents positions corresponding to those shown in Fig. 3(C). The Y-axis represents the fraction of aromatic amino acids (Y, W, F, H) at those positions in the respective families.
Similar articles
- Archaea: The Final Frontier of Chromatin.
Laursen SP, Bowerman S, Luger K. Laursen SP, et al. J Mol Biol. 2021 Mar 19;433(6):166791. doi: 10.1016/j.jmb.2020.166791. Epub 2020 Dec 29. J Mol Biol. 2021. PMID: 33383035 Free PMC article. Review. - DNA-binding proteins and evolution of transcription regulation in the archaea.
Aravind L, Koonin EV. Aravind L, et al. Nucleic Acids Res. 1999 Dec 1;27(23):4658-70. doi: 10.1093/nar/27.23.4658. Nucleic Acids Res. 1999. PMID: 10556324 Free PMC article. - The σ enigma: bacterial σ factors, archaeal TFB and eukaryotic TFIIB are homologs.
Burton SP, Burton ZF. Burton SP, et al. Transcription. 2014;5(4):e967599. doi: 10.4161/21541264.2014.967599. Transcription. 2014. PMID: 25483602 Free PMC article. - A prototypic lysine methyltransferase 4 from archaea with degenerate sequence specificity methylates chromatin proteins Sul7d and Cren7 in different patterns.
Niu Y, Xia Y, Wang S, Li J, Niu C, Li X, Zhao Y, Xiong H, Li Z, Lou H, Cao Q. Niu Y, et al. J Biol Chem. 2013 May 10;288(19):13728-40. doi: 10.1074/jbc.M113.452979. Epub 2013 Mar 25. J Biol Chem. 2013. PMID: 23530048 Free PMC article. - [Homologous protein domains in superkingdoms Archaea, Bacteria, and Eukaryota and the problem of the origin of eukaryotes].
Markov AV, Kulikov AM. Markov AV, et al. Izv Akad Nauk Ser Biol. 2005 Jul-Aug;(4):389-400. Izv Akad Nauk Ser Biol. 2005. PMID: 16212260 Review. Russian.
Cited by
- Global Analysis of the WOX Transcription Factor Family in Akebia trifoliata.
Chen S, Yang H, Zhang Y, Chen C, Ren T, Tan F, Luo P. Chen S, et al. Curr Issues Mol Biol. 2023 Dec 19;46(1):11-24. doi: 10.3390/cimb46010002. Curr Issues Mol Biol. 2023. PMID: 38275662 Free PMC article. - Mechanism and evolutionary origins of alanine-tail C-degron recognition by E3 ligases Pirh2 and CRL2-KLHDC10.
Patil PR, Burroughs AM, Misra M, Cerullo F, Costas-Insua C, Hung HC, Dikic I, Aravind L, Joazeiro CAP. Patil PR, et al. Cell Rep. 2023 Sep 26;42(9):113100. doi: 10.1016/j.celrep.2023.113100. Epub 2023 Sep 6. Cell Rep. 2023. PMID: 37676773 Free PMC article. - Archaea: The Final Frontier of Chromatin.
Laursen SP, Bowerman S, Luger K. Laursen SP, et al. J Mol Biol. 2021 Mar 19;433(6):166791. doi: 10.1016/j.jmb.2020.166791. Epub 2020 Dec 29. J Mol Biol. 2021. PMID: 33383035 Free PMC article. Review. - Dimerization of MORC2 through its C-terminal coiled-coil domain enhances chromatin dynamics and promotes DNA repair.
Xie HY, Zhang TM, Hu SY, Shao ZM, Li DQ. Xie HY, et al. Cell Commun Signal. 2019 Dec 3;17(1):160. doi: 10.1186/s12964-019-0477-5. Cell Commun Signal. 2019. PMID: 31796101 Free PMC article. - Effect of Human or Mouse IL-7 on the Homeostasis of Porcine T Lymphocytes.
Hong JH, Kim SH, Kim HG, Jang JH, Son RG, Pack SP, Park YH, Kang P, Jeong KJ, Kim JS, Choi H, Kim SU, Jung YW. Hong JH, et al. Immune Netw. 2021 Jun 25;21(3):e24. doi: 10.4110/in.2021.21.e24. eCollection 2021 Jun. Immune Netw. 2021. PMID: 34277114 Free PMC article.
References
- Schwede, T. & Peitsch, M. C. Computational Structural Biology: Methods and Applications. (World Scientific Publishing Company, 2008).
- Murzin AG, Brenner SE, Hubbard T, Chothia C. SCOP: a structural classification of proteins database for the investigation of sequences and structures. Journal of molecular biology. 1995;247:536–540. - PubMed
- Swindells MB, Orengo CA, Jones DT, Hutchinson EG, Thornton JM. Contemporary approaches to protein structure classification. BioEssays: news and reviews in molecular, cellular and developmental biology. 1998;20:884–891. doi: 10.1002/(SICI)1521-1878(199811)20:11<884::AID-BIES3>3.0.CO;2-H. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources