The perfect personalized cancer therapy: cancer vaccines against neoantigens - PubMed (original) (raw)
Review
The perfect personalized cancer therapy: cancer vaccines against neoantigens
Luigi Aurisicchio et al. J Exp Clin Cancer Res. 2018.
Abstract
In the advent of Immune Checkpoint inhibitors (ICI) and of CAR-T adoptive T-cells, the new frontier in Oncology is Cancer Immunotherapy because of its ability to provide long term clinical benefit in metastatic disease in several solid and liquid tumor types. It is now clear that ICI acts by unmasking preexisting immune responses as well as by inducing de novo responses against tumor neoantigens. Thanks to theprogress made in genomics technologies and the evolution of bioinformatics, neoantigens represent ideal targets, due to their specific expression in cancer tissue and the potential lack of side effects. In this review, we discuss the promise of preclinical and clinical results with mutation-derived neoantigen cancer vaccines (NCVs) along with the current limitations from bioinformatics prediction to manufacturing an effective new therapeutic approach.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
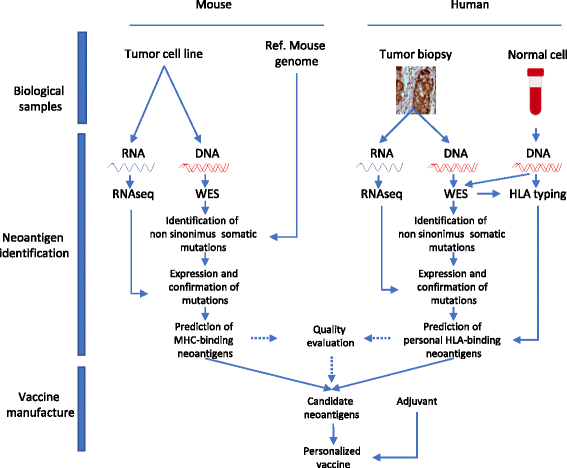
Fig. 1
The pipeline of neoantigen cancer vaccine production, for mouse studies (left side and human studies (right side). 1. Tumor and normal tissue are collected and subjected to (2) exome sequencing and RNAseq analysis for the tumor samples. 3. expressed non-synonymous mutations are then further selected according to binding to predictive algorithms and incorporated in a vaccine vector or delivered as peptides with adjuvants
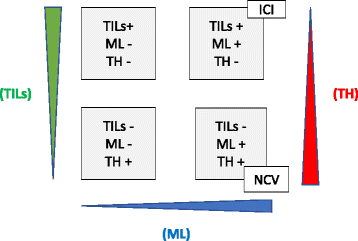
Fig. 2
Personalized NCV in the context of current immunotherapy, the three dimensions are defined by tumor infiltrating lymphocytes (TILs), mutational load (ML) and tumor heterogeneity (TH). Patients in the lower right panel (TIL−ML+TH+) may benefit from Neoantigen cancer vaccine (NCV) approach whereas patients in the upper right panel (TILs+ML+TH−) respond more to immune checkpoint inhibitors (ICI)
Similar articles
- Personalized neoantigen vaccines: A new approach to cancer immunotherapy.
Aldous AR, Dong JZ. Aldous AR, et al. Bioorg Med Chem. 2018 Jun 1;26(10):2842-2849. doi: 10.1016/j.bmc.2017.10.021. Epub 2017 Oct 19. Bioorg Med Chem. 2018. PMID: 29111369 Review. - Advances in personalized neoantigen vaccines for cancer immunotherapy.
Sun C, Xu S. Sun C, et al. Biosci Trends. 2020 Nov 4;14(5):349-353. doi: 10.5582/bst.2020.03267. Epub 2020 Sep 10. Biosci Trends. 2020. PMID: 32908077 Review. - Neoantigen identification strategies enable personalized immunotherapy in refractory solid tumors.
Chen F, Zou Z, Du J, Su S, Shao J, Meng F, Yang J, Xu Q, Ding N, Yang Y, Liu Q, Wang Q, Sun Z, Zhou S, Du S, Wei J, Liu B. Chen F, et al. J Clin Invest. 2019 Mar 5;129(5):2056-2070. doi: 10.1172/JCI99538. Print 2019 May 1. J Clin Invest. 2019. PMID: 30835255 Free PMC article. - Advances in the development of personalized neoantigen-based therapeutic cancer vaccines.
Blass E, Ott PA. Blass E, et al. Nat Rev Clin Oncol. 2021 Apr;18(4):215-229. doi: 10.1038/s41571-020-00460-2. Epub 2021 Jan 20. Nat Rev Clin Oncol. 2021. PMID: 33473220 Free PMC article. Review. - Personalized cancer neoantigen vaccines come of age.
Chu Y, Liu Q, Wei J, Liu B. Chu Y, et al. Theranostics. 2018 Jul 30;8(15):4238-4246. doi: 10.7150/thno.24387. eCollection 2018. Theranostics. 2018. PMID: 30128050 Free PMC article. Review.
Cited by
- Present and Future of Anti-Glioblastoma Therapies: A Deep Look into Molecular Dependencies/Features.
Kim HJ, Kim DY. Kim HJ, et al. Molecules. 2020 Oct 12;25(20):4641. doi: 10.3390/molecules25204641. Molecules. 2020. PMID: 33053763 Free PMC article. Review. - Immunotherapy in extensive small cell lung cancer.
Verma V, Sharma G, Singh A. Verma V, et al. Exp Hematol Oncol. 2019 Feb 4;8:5. doi: 10.1186/s40164-019-0129-x. eCollection 2019. Exp Hematol Oncol. 2019. PMID: 30740266 Free PMC article. Review. - Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer.
Picard E, Verschoor CP, Ma GW, Pawelec G. Picard E, et al. Front Immunol. 2020 Mar 6;11:369. doi: 10.3389/fimmu.2020.00369. eCollection 2020. Front Immunol. 2020. PMID: 32210966 Free PMC article. Review. - Artificial intelligence and neoantigens: paving the path for precision cancer immunotherapy.
Bulashevska A, Nacsa Z, Lang F, Braun M, Machyna M, Diken M, Childs L, König R. Bulashevska A, et al. Front Immunol. 2024 May 29;15:1394003. doi: 10.3389/fimmu.2024.1394003. eCollection 2024. Front Immunol. 2024. PMID: 38868767 Free PMC article. Review. - Harnessing Tumor Necrosis Factor Alpha to Achieve Effective Cancer Immunotherapy.
Mercogliano MF, Bruni S, Mauro F, Elizalde PV, Schillaci R. Mercogliano MF, et al. Cancers (Basel). 2021 Feb 2;13(3):564. doi: 10.3390/cancers13030564. Cancers (Basel). 2021. PMID: 33540543 Free PMC article. Review.
References
- Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov. 2017;7:264–276. doi: 10.1158/2159-8290.CD-16-0828. - DOI - PMC - PubMed
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjörd JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinski M, Jäger N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, López-Otín C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdés-Mas R, van Buuren MM, van 't Veer L, Vincent-Salomon A, Waddell N, Yates LR; Australian Pancreatic Cancer Genome Initiative; ICGC Breast Cancer Consortium; ICGC MMML-Seq Consortium; ICGC PedBrain, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. 10.1038/nature12477. Epub 2013 Aug 14. Erratum in: Nature. 2013 Oct 10;502(7470):258. Imielinsk, Marcin [corrected to Imielinski, Marcin]. - PMC - PubMed
- Zhao B, Sedlak JC, Srinivas R, Creixell P, Pritchard JR, Tidor B, et al. Exploiting temporal collateral sensitivity in tumor clonal evolution. Cell Elsevier Inc. 2016;165:234–46. Available from: http://www.cell.com/cell/fulltext/S0092-8674(16)30059-9 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources