Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective - PubMed (original) (raw)
Review
. 2018 Feb 26:2018:4178607.
doi: 10.1155/2018/4178607. eCollection 2018.
Affiliations
- PMID: 29682542
- PMCID: PMC5846367
- DOI: 10.1155/2018/4178607
Review
Gut Microbial Changes, Interactions, and Their Implications on Human Lifecycle: An Ageing Perspective
Ravichandra Vemuri et al. Biomed Res Int. 2018.
Abstract
Gut microbiota is established during birth and evolves with age, mostly maintaining the commensal relationship with the host. A growing body of clinical evidence suggests an intricate relationship between the gut microbiota and the immune system. With ageing, the gut microbiota develops significant imbalances in the major phyla such as the anaerobic Firmicutes and Bacteroidetes as well as a diverse range of facultative organisms, resulting in impaired immune responses. Antimicrobial therapy is commonly used for the treatment of infections; however, this may also result in the loss of normal gut flora. Advanced age, antibiotic use, underlying diseases, infections, hormonal differences, circadian rhythm, and malnutrition, either alone or in combination, contribute to the problem. This nonbeneficial gastrointestinal modulation may be reversed by judicious and controlled use of antibiotics and the appropriate use of prebiotics and probiotics. In certain persistent, recurrent settings, the option of faecal microbiota transplantation can be explored. The aim of the current review is to focus on the establishment and alteration of gut microbiota, with ageing. The review also discusses the potential role of gut microbiota in regulating the immune system, together with its function in healthy and diseased state.
Figures
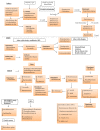
Figure 1
Overview of development of microbiota. The gastrointestinal tract (GI) is most sterile during the in utero stage. The first colonization happens based on mode of delivery either C-section or natural (vaginal delivery). Corynebacterium sp. is thought to be early colonizers in C-section and Lactobacillus sp. in the vaginal delivery. As the time progress the commensal bacterial community grows and is influenced by the solid food intake. During the initial stages of microbiota establishment the TLR receptor actions are minimal allowing growth of commensals. Eventually the immune system also grows by demarking the commensals and pathogens. Bacteroidetes domination begins after two years of birth. The relative stability is attained at the adulthood with Bacteroidetes and Firmicutes dominating. The alteration happens with use of antibiotics, obesity, GI orders, and diet. During elderly the relative stability declines, commensal community reduces, and pathogenic species like Clostridium increases. Malnutrition, alcohol abuse, decline in metabolism, frequent hospitalization, nosocomial infections (Clostridium difficile), and other pathogenic infections leading to Polypharmacy and ultimately to various inflammatory diseases.
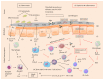
Figure 2
Interplay between immune system and gut microbiota in homeostasis, tolerance, and inflammation. (a) Both commensals and opportunists compete for the metabolites (SCFA) and various nutrients. The intestinal epithelial cells (IEC) play a role in steady state environment by releasing interleukins IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) factors in the presence of SCFA, PSA (Bact., fragilis), lipopolysaccharide (LPS), and AMPs (defensins, cathelicidins, and C-type lectins). IL-25, IL-33, and various growth related factors help in the transformation of progenitor basophils to basophils, activation of monocytes, macrophages, and mast cells functioning. The bacteriocins released by segmented filamentous bacteria (SFB) also influence the release of TSLP. The microfolding (M) cells upon sensing the presence of the microbes act as antigen presenting cell (APC) phagocytic activity by engulfing and presenting it to mucosal dendritic cells (DCs). In turn DCs are endowed with the ability to produce cytokines and other products such as IL-6 and IL-1_β_, tumor growth factor (TGF-β), retinoic acid (RA), and vitamin A. DCs form a major histocompatible complex (MHC) with the T cell receptors (TCR). In the presence of TGF-β and RA, the naïve T cells (CD4+ cells) transform into regulatory T cells (Treg). Simultaneously during interaction and competition of commensals and pathogens for nutrients, macrophages after recognition microbes released proinflammatory cytokine such as IL-10, which in turn helps with expansion of Treg cells which are already released during homeostasis and inflammation. Also with the help of DCs, macrophages release certain B cell activating factors which increase the production of secretory immunoglobulin A (SIgA) to maintain tolerance and steady state. (b) ((1) & (2)): in the elderly, there are declined physiological functioning and dysbiosis (reduction in commensal bacteria), resulting in an increase in pathogens. (3) The production of IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) by IEC reduces. (4) There is decline in M cell/APC activity to present PSA or microbe to DCs (activation of inflammatory pathway). Moreover lack of PSA stimulation reduces the IL-12 levels and releases T helper 2 cells. Decline in DCs not forming MHC with TCR reduces the population of active T cells such as Treg cells. Activation of B cells to plasma secretory cells and release of SIgA decreases. (5) The activation and function of macrophages (low levels of IL-10) are reduced. (6) The steady state or the tolerance is reduced. (7) Macrophages (inflammatory) are activated in the presence of pathogens and release proinflammatory cytokines (IL-1_β_, IL-6, and TNF-α) which leads to production of reactive oxygen species (ROS) and causes oxidative stress. Altogether with reduced levels of Treg and T helper cells and SIgA increase the pathogen invasion leading to release of proinflammatory cytokines and reduced anti-inflammatory cytokines increases inflammation, causing various GI disorders.
Similar articles
- Therapeutic interventions for gut dysbiosis and related disorders in the elderly: antibiotics, probiotics or faecal microbiota transplantation?
Vemuri RC, Gundamaraju R, Shinde T, Eri R. Vemuri RC, et al. Benef Microbes. 2017 Apr 26;8(2):179-192. doi: 10.3920/BM2016.0115. Epub 2016 Dec 23. Benef Microbes. 2017. PMID: 28008784 Review. - Role of the normal gut microbiota.
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Jandhyala SM, et al. World J Gastroenterol. 2015 Aug 7;21(29):8787-803. doi: 10.3748/wjg.v21.i29.8787. World J Gastroenterol. 2015. PMID: 26269668 Free PMC article. Review. - Symbiotic and antibiotic interactions between gut commensal microbiota and host immune system.
Malys MK, Campbell L, Malys N. Malys MK, et al. Medicina (Kaunas). 2015;51(2):69-75. doi: 10.1016/j.medici.2015.03.001. Epub 2015 Mar 24. Medicina (Kaunas). 2015. PMID: 25975874 Review. - Modulation of the gut microbiota: a focus on treatments for irritable bowel syndrome.
Harris LA, Baffy N. Harris LA, et al. Postgrad Med. 2017 Nov;129(8):872-888. doi: 10.1080/00325481.2017.1383819. Epub 2017 Oct 13. Postgrad Med. 2017. PMID: 28936910 Review. - Modulation of Gut Microbiota-Brain Axis by Probiotics, Prebiotics, and Diet.
Liu X, Cao S, Zhang X. Liu X, et al. J Agric Food Chem. 2015 Sep 16;63(36):7885-95. doi: 10.1021/acs.jafc.5b02404. Epub 2015 Sep 1. J Agric Food Chem. 2015. PMID: 26306709 Review.
Cited by
- 'Supporting the Support Staff': A Narrative Review of Nutritional Opportunities to Enhance Recovery and Wellbeing in Multi-Disciplinary Soccer Performance Staff.
Curtis C, Carling C, Tooley E, Russell M. Curtis C, et al. Nutrients. 2024 Oct 14;16(20):3474. doi: 10.3390/nu16203474. Nutrients. 2024. PMID: 39458469 Free PMC article. Review. - Gut microbial metabolites in MASLD: Implications of mitochondrial dysfunction in the pathogenesis and treatment.
Zhang R, Yan Z, Zhong H, Luo R, Liu W, Xiong S, Liu Q, Liu M. Zhang R, et al. Hepatol Commun. 2024 Jul 5;8(7):e0484. doi: 10.1097/HC9.0000000000000484. eCollection 2024 Jul 1. Hepatol Commun. 2024. PMID: 38967596 Free PMC article. Review. - From Gut Microbiota to Brain Waves: The Potential of the Microbiome and EEG as Biomarkers for Cognitive Impairment.
Krothapalli M, Buddendorff L, Yadav H, Schilaty ND, Jain S. Krothapalli M, et al. Int J Mol Sci. 2024 Jun 18;25(12):6678. doi: 10.3390/ijms25126678. Int J Mol Sci. 2024. PMID: 38928383 Free PMC article. Review. - Enterotype-Dependent Probiotic-Mediated Changes in the Male Rat Intestinal Microbiome In Vivo and In Vitro.
Kolzhetsov N, Markelova N, Frolova M, Alikina O, Glazunova O, Safonova L, Kalashnikova I, Yudin V, Makarov V, Keskinov A, Yudin S, Troshina D, Rechkina V, Shcherbakova V, Shavkunov K, Ozoline O. Kolzhetsov N, et al. Int J Mol Sci. 2024 Apr 22;25(8):4558. doi: 10.3390/ijms25084558. Int J Mol Sci. 2024. PMID: 38674145 Free PMC article. - Polyphenolic Compounds: Orchestrating Intestinal Microbiota Harmony during Aging.
Pereira QC, Fortunato IM, Oliveira FS, Alvarez MC, Santos TWD, Ribeiro ML. Pereira QC, et al. Nutrients. 2024 Apr 5;16(7):1066. doi: 10.3390/nu16071066. Nutrients. 2024. PMID: 38613099 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical