Ethanol-Induced Changes in PKCε: From Cell to Behavior - PubMed (original) (raw)
Review
Ethanol-Induced Changes in PKCε: From Cell to Behavior
Rashidi M Pakri Mohamed et al. Front Neurosci. 2018.
Abstract
The long-term binge intake of ethanol causes neuroadaptive changes that lead to drinkers requiring higher amounts of ethanol to experience its effects. This neuroadaptation can be partly attributed to the modulation of numerous neurotransmitter receptors by the various protein kinases C (PKCs). PKCs are enzymes that control cellular activities by regulating other proteins via phosphorylation. Among the various isoforms of PKC, PKCε is the most implicated in ethanol-induced biochemical and behavioral changes. Ethanol exposure causes changes to PKCε expression and localization in various brain regions that mediate addiction-favoring plasticity. Ethanol works in conjunction with numerous upstream kinases and second messenger activators to affect cellular PKCε expression. Chauffeur proteins, such as receptors for activated C kinase (RACKs), cause the translocation of PKCε to aberrant sites and mediate ethanol-induced changes. In this article, we aim to review the following: the general structure and function of PKCε, ethanol-induced changes in PKCε expression, the regulation of ethanol-induced PKCε activities in DAG-dependent and DAG-independent environments, the mechanisms underlying PKCε-RACKε translocation in the presence of ethanol, and the existing literature on the role of PKCε in ethanol-induced neurobehavioral changes, with the goal of creating a working model upon which further research can build.
Keywords: PKC; PKCε; RACK; alcohol; epsilon; ethanol.
Figures
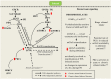
Figure 1
Signaling pathway of ethanol-mediated changes in PKCε activities (expression and translocation). Interaction of ethanol with GPCR results in generation of second messengers such as DAG and IP3. Long-term ethanol consumption upregulates cellular levels of basal PKCε and upstream kinases such as PI3K and mTORC2 which collectively increases phosohorylation of PKCε at S729. In DAG-dependent pathway, binding of DAG and RACK to mature PKCε translocates the kinase to distinct subcellular target sites to mediate downstream signaling pathways. In DAG-independent pathway, increase in DAG kinase activities causes generation of PA which could affect PKCε translocation via novel pathways. Once translocated, PKCε could affect downstream signaling through phosphorylation of numerous molecular targets which include GABAA, N-ethylmaleimide sensitif factor and mGlu5 which influence binge ethanol consumption. PKCε also causes internalization of mGlu5 surface receptors that could potentially reduce mGlu5 availability during abstinence.
Similar articles
- PKCepsilon activation induces dichotomous cardiac phenotypes and modulates PKCepsilon-RACK interactions and RACK expression.
Pass JM, Zheng Y, Wead WB, Zhang J, Li RC, Bolli R, Ping P. Pass JM, et al. Am J Physiol Heart Circ Physiol. 2001 Mar;280(3):H946-55. doi: 10.1152/ajpheart.2001.280.3.H946. Am J Physiol Heart Circ Physiol. 2001. PMID: 11179034 - Protein Kinase C Epsilon Activity in the Nucleus Accumbens and Central Nucleus of the Amygdala Mediates Binge Alcohol Consumption.
Cozzoli DK, Courson J, Rostock C, Campbell RR, Wroten MG, McGregor H, Caruana AL, Miller BW, Hu JH, Wu Zhang P, Xiao B, Worley PF, Crabbe JC, Finn DA, Szumlinski KK. Cozzoli DK, et al. Biol Psychiatry. 2016 Mar 15;79(6):443-51. doi: 10.1016/j.biopsych.2015.01.019. Epub 2015 Mar 5. Biol Psychiatry. 2016. PMID: 25861702 Free PMC article. - mTORC and PKCε in Regulation of Alcohol Use Disorder.
Hanim A, Mohamed IN, Mohamed RMP, Das S, Nor NSM, Harun RA, Kumar J. Hanim A, et al. Mini Rev Med Chem. 2020;20(17):1696-1708. doi: 10.2174/1389557520666200624122325. Mini Rev Med Chem. 2020. PMID: 32579497 Review. - Differential roles of PKC isoforms (PKCs) in GnRH stimulation of MAPK phosphorylation in gonadotrope derived cells.
Mugami S, Dobkin-Bekman M, Rahamim-Ben Navi L, Naor Z. Mugami S, et al. Mol Cell Endocrinol. 2018 Mar 5;463:97-105. doi: 10.1016/j.mce.2017.04.004. Epub 2017 Apr 6. Mol Cell Endocrinol. 2018. PMID: 28392410 - Protein kinase Cepsilon interacts with Stat3 and regulates its activation that is essential for the development of skin cancer.
Aziz MH, Manoharan HT, Sand JM, Verma AK. Aziz MH, et al. Mol Carcinog. 2007 Aug;46(8):646-53. doi: 10.1002/mc.20356. Mol Carcinog. 2007. PMID: 17583567 Review.
Cited by
- Interspecies and regional variability of alcohol action on large cerebral arteries: regulation by KCNMB1 proteins.
Mysiewicz S, North KC, Moreira L Jr, Odum SJ, Bukiya AN, Dopico AM. Mysiewicz S, et al. Am J Physiol Regul Integr Comp Physiol. 2023 Apr 1;324(4):R480-R496. doi: 10.1152/ajpregu.00103.2022. Epub 2023 Jan 30. Am J Physiol Regul Integr Comp Physiol. 2023. PMID: 36717168 Free PMC article. - Alcohol Use Disorder, Neurodegeneration, Alzheimer's and Parkinson's Disease: Interplay Between Oxidative Stress, Neuroimmune Response and Excitotoxicity.
Kamal H, Tan GC, Ibrahim SF, Shaikh MF, Mohamed IN, Mohamed RMP, Hamid AA, Ugusman A, Kumar J. Kamal H, et al. Front Cell Neurosci. 2020 Aug 31;14:282. doi: 10.3389/fncel.2020.00282. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33061892 Free PMC article. Review. - Leveraging genetic data to investigate molecular targets and drug repurposing candidates for treating alcohol use disorder and hepatotoxicity.
Gray JC, Murphy M, Leggio L. Gray JC, et al. Drug Alcohol Depend. 2020 Sep 1;214:108155. doi: 10.1016/j.drugalcdep.2020.108155. Epub 2020 Jul 2. Drug Alcohol Depend. 2020. PMID: 32652377 Free PMC article. - Nicotine and alcohol: the role of midbrain dopaminergic neurons in drug reinforcement.
Morel C, Montgomery S, Han MH. Morel C, et al. Eur J Neurosci. 2019 Aug;50(3):2180-2200. doi: 10.1111/ejn.14160. Epub 2018 Oct 15. Eur J Neurosci. 2019. PMID: 30251377 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources