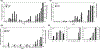Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation - an experimental study - PubMed (original) (raw)
. 2018 Oct;31(10):1164-1177.
doi: 10.1111/tri.13273. Epub 2018 Jun 5.
Tatsu Tanabe 2, Miguel A Lanaspa 1, Hironosuke Watanabe 2, Shunichiro Nomura 2, Ana Andres-Hernando 1, Krystle Garth 1, Mitsuhiro Sekijima 3, Takuji Ishimoto 1, Yuichi Ariyoshi 2, Gabriela E Garcia 1, Jigesh Shah 3, Boyd Lennan 2, Masayuki Tasaki 3, Thomas Pomposelli 2, Akira Shimizu 3, David H Sachs 2 3, Richard J Johnson 1, Kazuhiko Yamada 2
Affiliations
- PMID: 29722117
- PMCID: PMC6407427
- DOI: 10.1111/tri.13273
Upregulation of CD80 on glomerular podocytes plays an important role in development of proteinuria following pig-to-baboon xeno-renal transplantation - an experimental study
Christopher J Rivard et al. Transpl Int. 2018 Oct.
Abstract
We have previously reported that co-transplantation of the kidney with vascularized donor thymus from α-1,3-galactosyltransferase gene knockout pigs with an anti-CD154 with rituximab-based regimen led to improved xenograft survival in baboons with donor-specific unresponsiveness. However, nephrotic syndrome emerged as a complication in which the glomeruli showed mild mesangial expansion with similarities to minimal change disease (MCD) in humans. Since MCD is associated with CD80 expression in glomeruli and elevated urinary excretion, we evaluated a potential role for CD80 in xenograft nephropathy. Study 1 confirmed high urinary CD80 excretion in nephrotic animals with renal xenografts showing CD80 expression in glomeruli. In Study 2, baboons receiving xenografts received CTLA4-Ig once a week from the second postoperative week or no CTLA4-Ig. The non-CTLA4-Ig group developed severe proteinuria with modest mesangial expansion with high urinary excretion of CD80 and documented CD80 expression in glomerular podocytes. All of the recipients in non-CTLA4-Ig groups had to be euthanized before POD 60. In contrast, CTLA4-Ig group showed a marked reduction in proteinuria and survived significantly longer, up to 193 days. These results demonstrate that anti-CD80 targeted therapy represents a promising strategy for reduction of proteinuria following renal xeno-transplantation with improved survival.
Keywords: CD80; kidney transplantation; large animal model; proteinuria; xenograft.
© 2018 Steunstichting ESOT.
Conflict of interest statement
Conflicts of interest
The authors have declared no conflicts of interest.
Figures
Figure 1
Time course of urinary CD80 excretion relative to albuminuria in three baboons with porcine xenografts. Shown is the time course in postoperative days for baboon 289 (a), baboon 314 (b), and baboon 324 (c). A narrowed time course in these baboons shows urinary CD80 expression tended to increase in serial samples prior to increases in urine albumin excretion (d). Dark bars represent CD80 excretion (y bar on right) while urine albumin excretion is shown as open bars (y bar for albuminuria on left). *No sample was available.
Figure 2
(a) Anti-pig non-Gal antibodies assessed by FCM. Line graphs show median fluorescence index that was calculated by intensity at each point postoperative day (30, 40, 50, 60, 70, 80, and 90)/pre-Tx level. All baboons had preformed anti-non-Gal Nabs, IgM, and IgG, prior to thymokidney (TK) Tx that were adsorbed by TKs after revascularization. Anti-pig antibody levels never exceeded above pre-Tx levels (index less than 1), indicating no elicited anti-pig antibodies, IgM and IgG, developed after TK Tx in both CTLA4-Ig-treated (a-1) and CTLA4-Ig-nontreated (a-2) groups. (b) Baboon recipient survival days after porcine TK Tx in CTLA4-Ig group (B393, B394, 14P5) and non-CTLA4-IG group (B363, B341, B366, B358).
Figure 3
(a) Proteinuria assessed by dipstick in CTLA4-Ig group (a-1) and non-CTLA4-Ig group (a-2). Urine protein excretion was assessed three times a week. Proteinuria is described based on the following scale: 1+; 30 mg/dl, 2+; 100 mg/dl, 3+; 500 mg/dl, 4+; >500 mg/dl. (b) Western blot analysis of CD80 with molecular weight markers reveals CD80 to be 53 KDa. Also shown is the urinary excretion of CD80 in three recipients of TK that were treated with multiple doses of CTLA4-Ig (b-1: B394, B393, 14P5, b-1) and four recipients of TK without CTLA4-Ig therapy (b-2: B363, B341, B366, B358, b-2). Non-CTLA4-Ig-treated recipients consistently showed high urinary CD80 excretion following transplantation and greater proteinuria (b-2). In contrast, long-term survivors of TK with CTLA4Ig had many days with absent or minimal CD80 secretion and less proteinuria (b-1) although transient CD80 excretion was seen when nonimmunological complications such as ureteral obstruction or catheter complications developed.
Figure 4
CD80 Expression in a Nephrotic baboon. Baboon 314 developed severe nephrotic syndrome. CD80 was present in the glomeruli (c) but was negative in a normal porcine glomerulus (negative control, b), whereas it was positive in porcine spleen (a) [400×, CD80 in red, DAPI counterstain shows nuclei in blue].
Figure 5
CD80 expression in glomeruli of Thymokidney Grafts. The excised thymokidney graft at POD35 (B358, serum creatinine 0.8 mg/dl) appeared histologically normal (PAS. a-1) but CD80 upregulation was observed (a-2. red). (a3) Showed an image with anti-pig synaptopodin Ab alone (a-3. green). Double staining with anti-pig synaptopodin Ab (green) showed many CD80-positive cells which were double stained for synaptopodin (white arrows in a-4). Double staining for CD80 and CD31 was also performed. Rare CD31+ capillary endothelial cells (green) express CD80 (red) (double-positive cells were indicated by white arrow in a-5) of the thymokidney. Blue staining was DAPI. In contrast, a biopsy performed at POD 121 from baboon 14P5 which was not nephrotic with normal renal function (serum creatinine 0.8 mg/dl) showed a normal-appearing glomerulus (PAS. b-1), with no upregulation of CD80 by both single immunofluorescence staining (b-2) or double immunofluorescence staining (anti-CD80/anti-pig synaptopodin Ab, Fig. 4b4; anti-CD80/anti-CD31 ab, b-5) [4009, CD80, red; synaptopodin or CD31, green; DAPI, blue].
Figure 6
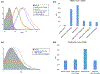
CD80 expression on porcine peripheral blood mononuclear cell (PBMC) and podocytes. (a) Pig PBMCs were stimulated with lipopolysaccharide (LPS) (5 μg/ml) for 24 h. CTLA4-Ig (at 0.25–5 μg/ml) was added and incubated for 3 h before LPS stimulation. CD80 expression was evaluated with flow cytometry (a-1). The mean MFI value in each of the groups was shown in the bar graph (a-2). Pig PBMC expressed CD80 (red line in the histogram, and third bar from left in the bar graph). CTLA4 Ig markedly inhibited CD80 expression. (b) Although resting pig podocytes did not express CD80 (blue line in the histogram and the second bar in the bar graph), LPS (5 μg/ml) stimulation induced CD80 upregulation (b-1, 2, red in histogram, and third bar from left in the bar graph). Pretreatment of CTLA4-Ig completely suppressed CD80 expression (green line in the histogram, and the fourth bar in the bar graph).
Figure 7
Replication of baboon CMV in sera of recipients in Study 2. (a) No bCMV replication was observed in all three baboons that were treated with CTLA4 Ig. (b) Three of four baboons in non-CTLA4-Ig-treated group did not develop any replication of bCMV by POD 20, and the remaining animal had only 19.4 copies/ml of serum at POD 10 and 242.95 copies/ml of serum at POD 20.
Similar articles
- Toll-like receptor 3 ligand, polyIC, induces proteinuria and glomerular CD80, and increases urinary CD80 in mice.
Ishimoto T, Shimada M, Gabriela G, Kosugi T, Sato W, Lee PY, Lanaspa MA, Rivard C, Maruyama S, Garin EH, Johnson RJ. Ishimoto T, et al. Nephrol Dial Transplant. 2013 Jun;28(6):1439-46. doi: 10.1093/ndt/gfs543. Epub 2012 Dec 21. Nephrol Dial Transplant. 2013. PMID: 23262434 Free PMC article. - Expression of human CD47 in pig glomeruli prevents proteinuria and prolongs graft survival following pig-to-baboon xenotransplantation.
Takeuchi K, Ariyoshi Y, Shimizu A, Okumura Y, Cara-Fuentes G, Garcia GE, Pomposelli T, Watanabe H, Boyd L, Ekanayake-Alper DK, Amarnath D, Sykes M, Sachs DH, Johnson RJ, Yamada K. Takeuchi K, et al. Xenotransplantation. 2021 Nov;28(6):e12708. doi: 10.1111/xen.12708. Epub 2021 Aug 21. Xenotransplantation. 2021. PMID: 34418164 Free PMC article. - Rituximab treatment prevents the early development of proteinuria following pig-to-baboon xeno-kidney transplantation.
Tasaki M, Shimizu A, Hanekamp I, Torabi R, Villani V, Yamada K. Tasaki M, et al. J Am Soc Nephrol. 2014 Apr;25(4):737-44. doi: 10.1681/ASN.2013040363. Epub 2014 Jan 23. J Am Soc Nephrol. 2014. PMID: 24459229 Free PMC article. - CTLA4-Ig in B7-1-positive diabetic and non-diabetic kidney disease.
Bassi R, Fornoni A, Doria A, Fiorina P. Bassi R, et al. Diabetologia. 2016 Jan;59(1):21-29. doi: 10.1007/s00125-015-3766-6. Epub 2015 Sep 26. Diabetologia. 2016. PMID: 26409459 Free PMC article. Review. - Abatacept and Glomerular Diseases: The Open Road for the Second Signal as a New Target is Settled Down.
Trimarchi H. Trimarchi H. Recent Pat Endocr Metab Immune Drug Discov. 2015;9(1):2-14. doi: 10.2174/1872214809666150302104542. Recent Pat Endocr Metab Immune Drug Discov. 2015. PMID: 25733062 Review.
Cited by
- Pretransplant Screening for Prevention of Hyperacute Graft Loss in Pig-to-primate Kidney Xenotransplantation.
Hisadome Y, Eisenson DL, Santillan MR, Iwase H, Yamada K. Hisadome Y, et al. Transplantation. 2024 Aug 1;108(8):1749-1759. doi: 10.1097/TP.0000000000004958. Epub 2024 Jul 20. Transplantation. 2024. PMID: 39042769 - Physiological basis for xenotransplantation from genetically modified pigs to humans.
Peterson L, Yacoub MH, Ayares D, Yamada K, Eisenson D, Griffith BP, Mohiuddin MM, Eyestone W, Venter JC, Smolenski RT, Rothblatt M. Peterson L, et al. Physiol Rev. 2024 Jul 1;104(3):1409-1459. doi: 10.1152/physrev.00041.2023. Epub 2024 Mar 22. Physiol Rev. 2024. PMID: 38517040 Free PMC article. Review. - Combined islet and kidney xenotransplantation for diabetic nephropathy: an update in ongoing research for a clinically relevant application of porcine islet transplantation.
Eisenson DL, Iwase H, Chen W, Hisadome Y, Cui W, Santillan MR, Schulick AC, Gu D, Maxwell A, Koenig K, Sun Z, Warren D, Yamada K. Eisenson DL, et al. Front Immunol. 2024 Feb 27;15:1351717. doi: 10.3389/fimmu.2024.1351717. eCollection 2024. Front Immunol. 2024. PMID: 38476227 Free PMC article. Review. - Progress in islet xenotransplantation: Immunologic barriers, advances in gene editing, and tolerance induction strategies for xenogeneic islets in pig-to-primate transplantation.
Eisenson DL, Hisadome Y, Santillan MR, Yamada K. Eisenson DL, et al. Front Transplant. 2022;1:989811. doi: 10.3389/frtra.2022.989811. Epub 2022 Oct 20. Front Transplant. 2022. PMID: 38390384 Free PMC article. - Chimeric Livers: Interspecies Blastocyst Complementation and Xenotransplantation for End-Stage Liver Disease.
Blake MJ, Steer CJ. Blake MJ, et al. Hepat Med. 2024 Feb 16;16:11-29. doi: 10.2147/HMER.S440697. eCollection 2024. Hepat Med. 2024. PMID: 38379783 Free PMC article. Review.
References
- Yamada K, Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med 2005; 11: 32. - PubMed
- Kuwaki K, Tseng YL, Dor FJ, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med 2005; 11: 29. - PubMed
- Yamada K, Sachs DH, DerSimonian H. Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J Immunol 1995; 155: 5249. - PubMed
MeSH terms
Substances
Grants and funding
- Bristol-Myers Squibb/International
- 2P01AI45897/GF/NIH HHS/United States
- P01 AI045897/AI/NIAID NIH HHS/United States
- T32 HL007854/HL/NHLBI NIH HHS/United States
- 6PO1AI45897/GF/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical