Mutations in C11orf70 Cause Primary Ciliary Dyskinesia with Randomization of Left/Right Body Asymmetry Due to Defects of Outer and Inner Dynein Arms - PubMed (original) (raw)
. 2018 May 3;102(5):973-984.
doi: 10.1016/j.ajhg.2018.03.025.
Rim Hjeij 1, Heike Olbrich 1, Gerard W Dougherty 1, Tabea Nöthe-Menchen 1, Isabella Aprea 1, Diana Frank 1, Petra Pennekamp 1, Bernd Dworniczak 1, Julia Wallmeier 1, Johanna Raidt 1, Kim G Nielsen 2, Maria C Philipsen 2, Francesca Santamaria 3, Laura Venditto 3, Israel Amirav 4, Huda Mussaffi 5, Freerk Prenzel 6, Kaman Wu 7, Zeineb Bakey 7, Miriam Schmidts 8, Niki T Loges 1, Heymut Omran 9
Affiliations
- PMID: 29727693
- PMCID: PMC5986731
- DOI: 10.1016/j.ajhg.2018.03.025
Mutations in C11orf70 Cause Primary Ciliary Dyskinesia with Randomization of Left/Right Body Asymmetry Due to Defects of Outer and Inner Dynein Arms
Inga M Höben et al. Am J Hum Genet. 2018.
Abstract
Primary ciliary dyskinesia (PCD) is characterized by chronic airway disease, male infertility, and randomization of the left/right body axis as a result of defects of motile cilia and sperm flagella. We identified loss-of-function mutations in the open-reading frame C11orf70 in PCD individuals from five distinct families. Transmission electron microscopy analyses and high-resolution immunofluorescence microscopy demonstrate that loss-of-function mutations in C11orf70 cause immotility of respiratory cilia and sperm flagella, respectively, as a result of the loss of axonemal outer (ODAs) and inner dynein arms (IDAs), indicating that C11orf70 is involved in cytoplasmic assembly of dynein arms. Expression analyses of C11orf70 showed that C11orf70 is expressed in ciliated respiratory cells and that the expression of C11orf70 is upregulated during ciliogenesis, similar to other previously described cytoplasmic dynein-arm assembly factors. Furthermore, C11orf70 shows an interaction with cytoplasmic ODA/IDA assembly factor DNAAF2, supporting our hypothesis that C11orf70 is a preassembly factor involved in the pathogenesis of PCD. The identification of additional genetic defects that cause PCD and male infertility is of great importance for the clinic as well as for genetic counselling.
Keywords: C11ORF70; cilia; dynein arms; preassembly; primary ciliary dyskinesia; sperm flagella.
Copyright © 2018 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
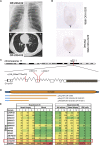
Recessive Loss-of-Function Mutations in C11orf70 Cause Primary Ciliary Dyskinesia with Randomization of Left/Right Body Asymmetry (A) The chest X-ray radiograph of PCD-affected individual OP-2334 II2 depicts situs inversus totalis. The computed tomography scan of OP-2334 II2 shows chronic airway disease with bronchiectasis in the middle lobe and mucus plugging. (B) In situ hybridization analyses of wild-type 8.25 dpc (days post-coitum) mouse embryos reveal expression of 9230110C19Rik (C11orf70 ortholog) exclusively at the left/right organizer (frontal view; asterisks in the ventral position of the left/right organizer). By contrast, the negative control utilizing the sense probe does not show any staining. (C) Schematic presentation of chromosome 11 and the exon-intron structure of C11orf70 with untranslated (gray) and translated (white) regions. The positions of the three identified mutations in the five unrelated families are indicated by red lines. (D) Protein model of C11orf70 with the domain of unknown function 4498 (DUF 4498) and the predicted truncated proteins as consequences of the three C11orf70 loss-of-function mutations. (E) Differentiation-specific and tissue-specific expression profiles of known genes encoding proteins involved in outer- and inner-dynein-arm assembly and GAPDH as a housekeeping gene. Raw RNA-seq data were normalized against PPIH (peptidylprolyl isomerase H). The preassembly-factor genes have the strongest expression in native material of nasal-brushing biopsies. Comparable expression profiles are observed in ALI-cultured nasal epithelial cells and in nasal-brushing biopsies. EBV cells (immortalized lymphocytes) and blood cells show no or weak expression of genes encoding cytoplasmic dynein assembly factors. GAPDH shows a high expression in all analyzed tissues. We performed two independent experiments with samples from five control individuals (samples 1–5).
Figure 2
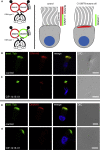
_C11orf70_-Mutant Respiratory Ciliary Axonemes Exhibit Ultrastructural Defects of the Outer and Inner Dynein Arms (A) Schematic diagram of a cross-section through a motile respiratory 9 + 2 cilium. (B) Arrangement of ODAs (blue) and IDAs (green) within the 96 nm unit in human ciliary axonemes. Whereas ODAs occur once every 24 nm within the 96 nm unit, the double-headed IDA complex I1/f, which contains the α- and β-heavy chain and the single-headed complexes (containing the dyneins a–d and g) are distributed with a 96 nm periodicity. (C) Transmission electron-microscopy photographs of cross-sections through respiratory epithelial cilia demonstrate the absence of ODAs and IDAs in PCD-affected individuals OI-87, OP-1416 II1, OP-2190, OP-2249 II1, and OP-2334 II2 carrying biallelic C11orf70 mutations, which was in contrast to a control cilium. The scale bar represents 200 nm. Abbreviations are as follows: CP, central pair; CS, central sheath; IDA, inner dynein arm; MT A, microtubule A; MT B, microtubule B; N-DRC, nexin-dynein regulatory complex; ODA, outer dynein arm; and RS, radial spoke.
Figure 3
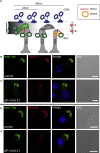
Absence of the Outer-Dynein-Arm Heavy Chains DNAH5 and DNAH9 in Respiratory Cilia of PCD-Affected Individuals Carrying C11orf70 Mutations (A) Schematics of type 1 and 2 ODAs show that DNAH11 is a component of type 1 ODA and localizes only to the proximal part, whereas DNAH9 is a component of type 2 ODA and localizes only to the distal part of the ciliary axonemes. DNAH5 is a component of both ODA types and localizes throughout the ciliary axoneme. In _C11orf70_-mutant cilia, DNAH5 and DNAH9 are absent from the ciliary axonemes, whereas DNAH11 localization is not affected by mutations in C11orf70 (Figure S8). (B and C) Respiratory cilia double-labeled with antibodies directed against acetylated α-tubulin (green) and DNAH5 (red) show colocalization of DNAH5 with acetylated α-tubulin along the cilia from unaffected controls (B, yellow). In contrast, DNAH5 is absent or severely reduced in _C11orf70_-mutant axonemes (C, shown for OP-1416 II1). Please note that the red signal at the ciliary base in (C) is an unspecific additional staining caused by rabbit polyclonal antibodies (see References for further details). (D and E) Respiratory epithelial cells from control and PCD-affected individuals were double-labeled with antibodies directed against acetylated tubulin (green) and DNAH9 (red). Acetylated α-tubulin localizes to the entire length of the ciliary axoneme, whereas DNAH9 localization is restricted to the distal part of the axoneme in healthy control cells (D). In _C11orf70_-mutant cells, DNAH9 is absent from the ciliary axonemes (E, shown for OP-1416 II1). Nuclei were stained with Hoechst33342 (blue). Scale bars represent 10 μm.
Figure 4
In _C11orf70_-Mutant Respiratory Cilia the IDA Light Chain DNALI1 (IDA-group I2) as Well as the IDA Heavy Chain DNAH6 (IDA-group I3) Are Absent (A) Schematic of the arrangement of ODAs (blue) and IDAs (green) within the 96 nm unit in the human ciliary axoneme. IDA complexes a, c, and d belong to the IDA group I2, characterized by the presence of the dynein light chain DNALI1 (red lines). IDA complex g contains the dynein heavy chain DNAH6 (yellow circle) and belongs to the IDA group I3. (B and C) Respiratory cilia double labeled with antibodies directed against acetylated α-tubulin (green) and DNALI1 (red) show colocalization of DNALI1 with acetylated α-tubulin along the cilia from unaffected controls (B, yellow). In contrast, DNALI1 is absent or severely reduced in _C11orf70_-mutant axonemes (C, OP-1416 II1). Please note that the red signal at the ciliary base in (C) is an unspecific additional staining caused by rabbit polyclonal antibodies (see References for further details). (D and E) Respiratory epithelial cells from control and PCD-affected individuals were double labeled with antibodies directed against acetylated α-tubulin (green) and DNAH6 (red). DNAH6 colocalizes with acetylated tubulin along the cilia from unaffected controls (D, yellow). In contrast, DNAH6 is absent or severely reduced in _C11orf70_-mutant axonemes (E, OP-1416 II1). Nuclei were stained with Hoechst33342 (blue). Scale bars represent 10 μm.
Figure 5
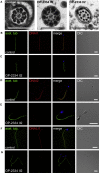
C11orf70 Is Necessary for Correct Assembly of Outer and Inner Dynein Arms in Sperm Flagella (A) TEM photographs of cross-sections of sperm flagella from a healthy control individual and from OP-2334 II2. The outer and inner dynein arms are present in control sperm flagella but are absent from the sperm flagella of OP-2334 II2. The scale bar represents 500 nm. (B–G) To confirm the ultrastructural defects of the dynein arms, we double-labeled sperm from an unaffected control (B, D, and F) and OP-2334 II2 (C, E, and G) with antibodies directed against acetylated tubulin (green) and either ODA proteins (DNAI1 and DNAI2) or IDA protein (DNALI1) (red). DNAI1, DNAI2 and DNALI1 colocalize with acetylated tubulin along the sperm flagellum from the unaffected control (B, D, and F, yellow). In contrast, DNAI1, DNAI2, and DNALI1 are absent or severely reduced in _C11orf70_-mutant sperm flagella (C, E, and G). Nuclei were stained with Hoechst33342 (blue). Scale bars represent 10 μm.
Similar articles
- Defects in the cytoplasmic assembly of axonemal dynein arms cause morphological abnormalities and dysmotility in sperm cells leading to male infertility.
Aprea I, Raidt J, Höben IM, Loges NT, Nöthe-Menchen T, Pennekamp P, Olbrich H, Kaiser T, Biebach L, Tüttelmann F, Horvath J, Schubert M, Krallmann C, Kliesch S, Omran H. Aprea I, et al. PLoS Genet. 2021 Feb 26;17(2):e1009306. doi: 10.1371/journal.pgen.1009306. eCollection 2021 Feb. PLoS Genet. 2021. PMID: 33635866 Free PMC article. - C11orf70 Mutations Disrupting the Intraflagellar Transport-Dependent Assembly of Multiple Axonemal Dyneins Cause Primary Ciliary Dyskinesia.
Fassad MR, Shoemark A, le Borgne P, Koll F, Patel M, Dixon M, Hayward J, Richardson C, Frost E, Jenkins L, Cullup T, Chung EMK, Lemullois M, Aubusson-Fleury A, Hogg C, Mitchell DR, Tassin AM, Mitchison HM. Fassad MR, et al. Am J Hum Genet. 2018 May 3;102(5):956-972. doi: 10.1016/j.ajhg.2018.03.024. Am J Hum Genet. 2018. PMID: 29727692 Free PMC article. - Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia.
Moore DJ, Onoufriadis A, Shoemark A, Simpson MA, zur Lage PI, de Castro SC, Bartoloni L, Gallone G, Petridi S, Woollard WJ, Antony D, Schmidts M, Didonna T, Makrythanasis P, Bevillard J, Mongan NP, Djakow J, Pals G, Lucas JS, Marthin JK, Nielsen KG, Santoni F, Guipponi M, Hogg C, Antonarakis SE, Emes RD, Chung EM, Greene ND, Blouin JL, Jarman AP, Mitchison HM. Moore DJ, et al. Am J Hum Genet. 2013 Aug 8;93(2):346-56. doi: 10.1016/j.ajhg.2013.07.009. Epub 2013 Jul 25. Am J Hum Genet. 2013. PMID: 23891471 Free PMC article. - Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome.
Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, Knowles MR, Zariwala MA. Leigh MW, et al. Genet Med. 2009 Jul;11(7):473-87. doi: 10.1097/GIM.0b013e3181a53562. Genet Med. 2009. PMID: 19606528 Free PMC article. Review. - Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure.
Shapiro AJ, Leigh MW. Shapiro AJ, et al. Ultrastruct Pathol. 2017 Nov-Dec;41(6):373-385. doi: 10.1080/01913123.2017.1362088. Epub 2017 Sep 15. Ultrastruct Pathol. 2017. PMID: 28915070 Free PMC article. Review.
Cited by
- Emerging Genotype-Phenotype Relationships in Primary Ciliary Dyskinesia.
Brennan SK, Ferkol TW, Davis SD. Brennan SK, et al. Int J Mol Sci. 2021 Jul 31;22(15):8272. doi: 10.3390/ijms22158272. Int J Mol Sci. 2021. PMID: 34361034 Free PMC article. Review. - Role of the Novel Hsp90 Co-Chaperones in Dynein Arms' Preassembly.
Fabczak H, Osinka A. Fabczak H, et al. Int J Mol Sci. 2019 Dec 7;20(24):6174. doi: 10.3390/ijms20246174. Int J Mol Sci. 2019. PMID: 31817850 Free PMC article. Review. - Schmidtea mediterranea as a Model Organism to Study the Molecular Background of Human Motile Ciliopathies.
Rabiasz A, Ziętkiewicz E. Rabiasz A, et al. Int J Mol Sci. 2023 Feb 24;24(5):4472. doi: 10.3390/ijms24054472. Int J Mol Sci. 2023. PMID: 36901899 Free PMC article. Review. - TTC12 Loss-of-Function Mutations Cause Primary Ciliary Dyskinesia and Unveil Distinct Dynein Assembly Mechanisms in Motile Cilia Versus Flagella.
Thomas L, Bouhouche K, Whitfield M, Thouvenin G, Coste A, Louis B, Szymanski C, Bequignon E, Papon JF, Castelli M, Lemullois M, Dhalluin X, Drouin-Garraud V, Montantin G, Tissier S, Duquesnoy P, Copin B, Dastot F, Couvet S, Barbotin AL, Faucon C, Honore I, Maitre B, Beydon N, Tamalet A, Rives N, Koll F, Escudier E, Tassin AM, Touré A, Mitchell V, Amselem S, Legendre M. Thomas L, et al. Am J Hum Genet. 2020 Feb 6;106(2):153-169. doi: 10.1016/j.ajhg.2019.12.010. Epub 2020 Jan 23. Am J Hum Genet. 2020. PMID: 31978331 Free PMC article. - Ciliary Dyneins and Dynein Related Ciliopathies.
Antony D, Brunner HG, Schmidts M. Antony D, et al. Cells. 2021 Jul 25;10(8):1885. doi: 10.3390/cells10081885. Cells. 2021. PMID: 34440654 Free PMC article. Review.
References
- Fliegauf M., Benzing T., Omran H. When cilia go bad: Cilia defects and ciliopathies. Nat. Rev. Mol. Cell Biol. 2007;8:880–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases