Old Drugs as New Treatments for Neurodegenerative Diseases - PubMed (original) (raw)
Review
Old Drugs as New Treatments for Neurodegenerative Diseases
Fernando Durães et al. Pharmaceuticals (Basel). 2018.
Abstract
Neurodegenerative diseases are increasing in number, given that the general global population is becoming older. They manifest themselves through mechanisms that are not fully understood, in many cases, and impair memory, cognition and movement. Currently, no neurodegenerative disease is curable, and the treatments available only manage the symptoms or halt the progression of the disease. Therefore, there is an urgent need for new treatments for this kind of disease, since the World Health Organization has predicted that neurodegenerative diseases affecting motor function will become the second-most prevalent cause of death in the next 20 years. New therapies can come from three main sources: synthesis, natural products, and existing drugs. This last source is known as drug repurposing, which is the most advantageous, since the drug’s pharmacokinetic and pharmacodynamic profiles are already established, and the investment put into this strategy is not as significant as for the classic development of new drugs. There have been several studies on the potential of old drugs for the most relevant neurodegenerative diseases, including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, Multiple Sclerosis and Amyotrophic Lateral Sclerosis.
Keywords: Alzheimer’s disease; Huntington’s disease; Parkinson’s disease; amyotrophic lateral sclerosis; drug repurposing; multiple sclerosis; neurodegenerative diseases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
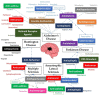
Figure 1
Summary of the diseases and repurposed drugs presented in this review.
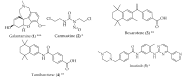
Figure 2
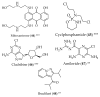
Structure of drugs 1–29 (*) Preclinical Studies; (**) Under clinical studies; (***) Clinically approved.
Figure 2
Structure of drugs 1–29 (*) Preclinical Studies; (**) Under clinical studies; (***) Clinically approved.
Figure 2
Structure of drugs 1–29 (*) Preclinical Studies; (**) Under clinical studies; (***) Clinically approved.
Figure 2
Structure of drugs 1–29 (*) Preclinical Studies; (**) Under clinical studies; (***) Clinically approved.
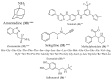
Figure 3
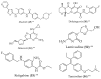
Structure of drugs 30–36 (*) Preclinical Studies; (**) Under clinical studies; (***) Clinically approved.
Figure 4
Structure of drugs 37–43 (*) Preclinical Studies; (**) Under clinical studies; (***) Clinically approved.
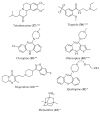
Figure 5
Structure of drugs 44–48 (*) Preclinical Studies; (**) Under clinical studies; (***) Clinically approved.
Figure 6
Structure of drugs 49–54 (*) Preclinical Studies; (**) Under clinical studies; (***) Clinically approved.
Similar articles
- Repurposing small-molecule drugs for modulating toxic protein aggregates in neurodegenerative diseases.
Liu W, Wang G, Wang Z, Wang G, Huang J, Liu B. Liu W, et al. Drug Discov Today. 2022 Jul;27(7):1994-2007. doi: 10.1016/j.drudis.2022.04.003. Epub 2022 Apr 6. Drug Discov Today. 2022. PMID: 35395400 Review. - The potential of epigenetic therapies in neurodegenerative diseases.
Coppedè F. Coppedè F. Front Genet. 2014 Jul 14;5:220. doi: 10.3389/fgene.2014.00220. eCollection 2014. Front Genet. 2014. PMID: 25071843 Free PMC article. Review. - The role of natural products in the discovery of new drug candidates for the treatment of neurodegenerative disorders I: Parkinson's disease.
Campos HC, da Rocha MD, Viegas FP, Nicastro PC, Fossaluzza PC, Fraga CA, Barreiro EJ, Viegas C Jr. Campos HC, et al. CNS Neurol Disord Drug Targets. 2011 Mar;10(2):239-50. doi: 10.2174/187152711794480483. CNS Neurol Disord Drug Targets. 2011. PMID: 20874702 Review. - The Potential of Flavonoids for the Treatment of Neurodegenerative Diseases.
Maher P. Maher P. Int J Mol Sci. 2019 Jun 22;20(12):3056. doi: 10.3390/ijms20123056. Int J Mol Sci. 2019. PMID: 31234550 Free PMC article. Review. - 2,4 Dinitrophenol as Medicine.
Geisler JG. Geisler JG. Cells. 2019 Mar 23;8(3):280. doi: 10.3390/cells8030280. Cells. 2019. PMID: 30909602 Free PMC article. Review.
Cited by
- Identification and Characterization of Neuroprotective Properties of Thaumatin-like Protein 1a from Annurca Apple Flesh Polyphenol Extract.
D'Errico A, Nasso R, Di Maro A, Landi N, Chambery A, Russo R, D'Angelo S, Masullo M, Arcone R. D'Errico A, et al. Nutrients. 2024 Jan 19;16(2):307. doi: 10.3390/nu16020307. Nutrients. 2024. PMID: 38276545 Free PMC article. - Calabria as a Genetic Isolate: A Model for the Study of Neurodegenerative Diseases.
Bruno F, Laganà V, Di Lorenzo R, Bruni AC, Maletta R. Bruno F, et al. Biomedicines. 2022 Sep 15;10(9):2288. doi: 10.3390/biomedicines10092288. Biomedicines. 2022. PMID: 36140389 Free PMC article. Review. - Synergistic effects of plasma-activated medium in combination with Baicalin against neuronal damage.
Zhu J, Liu Q, Chen Y, Zhang J, Xu Q, Wu Z. Zhu J, et al. Heliyon. 2024 Aug 11;10(16):e36079. doi: 10.1016/j.heliyon.2024.e36079. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39224291 Free PMC article. - Neurohormetic phytochemicals in the pathogenesis of neurodegenerative diseases.
Sahebnasagh A, Eghbali S, Saghafi F, Sureda A, Avan R. Sahebnasagh A, et al. Immun Ageing. 2022 Aug 11;19(1):36. doi: 10.1186/s12979-022-00292-x. Immun Ageing. 2022. PMID: 35953850 Free PMC article. Review. - Neuroimmunometabolism: A New Pathological Nexus Underlying Neurodegenerative Disorders.
Mitra S, Banik A, Saurabh S, Maulik M, Khatri SN. Mitra S, et al. J Neurosci. 2022 Mar 9;42(10):1888-1907. doi: 10.1523/JNEUROSCI.0998-21.2022. Epub 2022 Jan 13. J Neurosci. 2022. PMID: 35027409 Free PMC article. Review.
References
- Doan T.L., Pollastri M., Walters M.A., Georg G.I. Chapter 23—The future of drug repositioning: Old drugs, new opportunities. In: Macor J.E., editor. Annual Reports in Medicinal Chemistry. Volume 46. Academic Press; Waltham, MA, USA: 2011. pp. 385–401.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources