Physical interaction of STAT1 isoforms with TGF-β receptors leads to functional crosstalk between two signaling pathways in epithelial ovarian cancer - PubMed (original) (raw)
Physical interaction of STAT1 isoforms with TGF-β receptors leads to functional crosstalk between two signaling pathways in epithelial ovarian cancer
Xiaoling Tian et al. J Exp Clin Cancer Res. 2018.
Abstract
Background: The signal transducer and activator of transcription (STAT) and transforming growth factor-β (TGF-β) signaling pathways play important roles in epithelial ovarian cancer (EOC). However, the mechanism of crosstalk between two pathways is not completely understood.
Methods: The expression of STAT1 protein was detected by tissue microarray and immunoblotting (IB). The interaction of STAT1 isoforms with TGF-β receptors was confirmed by immunoprecipitation and IB. The effect of TGF-β signaling on STAT1 activation was examined in EOC and non-tumorous HOSEpiC cells treated with TGF-β1 in the presence or absence of the inhibitor of TGF-β type I receptor. The gain-of-function and loss-of-function approaches were applied for detecting the role of STAT1 on EOC cell behaviours.
Results: The high level of STAT1 was observed in patients with high-grade serous EOC. STAT1 expression was higher in ovarian cancer cells than noncancerous cells. TGF-β1 activated the STAT1 pathway by inducing the phosphorylation of STAT1α on S727 residue. The full-length STAT1α and the truncated STAT1β directly interacted with TGF-β receptors (ALK1/ALK5 and TβRII), which was mediated by TGF-β1. STAT1α and STAT1β blocked the activation of the TGF-β1 signaling pathway in EOC cells by reducing Smad2 phosphorylation. STAT1 overexpression induced EOC cell proliferation, migration, and invasion; whereas its inhibition enhanced TGF-β1-induced phospho-Smad2 and suppressed EOC cell proliferation, migration, and invasion.
Conclusions: Our data unveil a novel insight into the molecular mechanism of crosstalk between the STAT1 and TGF-β signaling pathways, which affected the cancer cell behavior. Suppression of STAT1 may be a potential therapeutic strategy for targeting ovarian cancer.
Keywords: Activin receptor-like kinase; Cell proliferation; Crosstalk; Cytokine; Growth factor; Receptor; Signal transduction; Smad; Tumorigenesis; TβRII.
Conflict of interest statement
Ethics approval and consent to participate
The study on human subjects was approved by the Ethics Committee of Jinshan Hospital, Fudan University (Reference # 2013–019-01).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Fig. 1
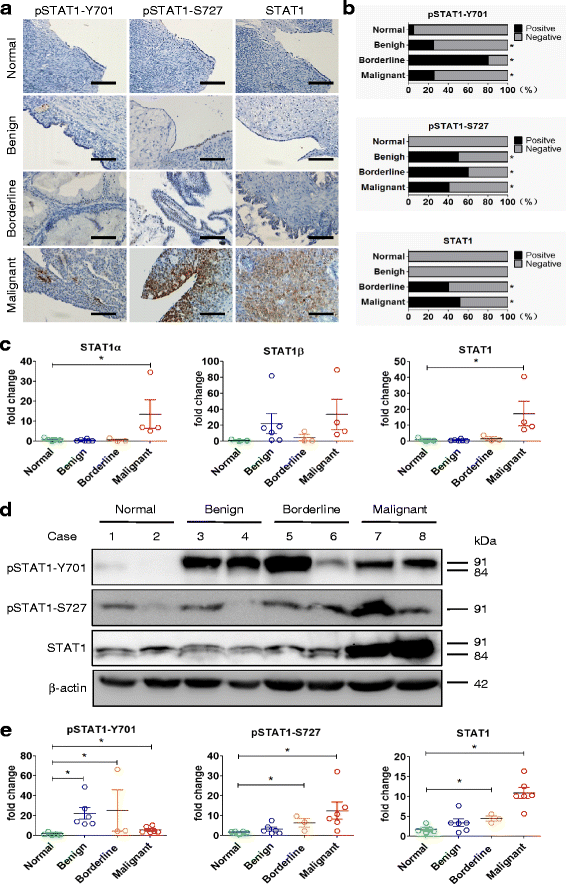
STAT1 is overexpressed in human epithelial ovarian cancer. a Immunohistochemical staining of STAT1 in ovarian tissues. A brown color in epithelial cells is considered as a positive staining. Representative images of pSTAT1-Y701, pSTAT1-S727, and total STAT1 expression in normal ovarian tissue (Normal), benign tumor (Benign), serous borderline tumor (Borderline), and high-grade serous carcinoma (Malignant) are shown. Original magnification, × 400; scale bar, 100 μm. b The case rate of pSTAT1-Y701, pSTAT1-S727, and STAT1 positivity and negativity. For comparison between two groups, χ2 test was applied. c STAT1 mRNA expression detected by quantitative RT-PCR. The expression of STAT1α and total STAT1 was higher in ovarian serous malignant tumors (Normal, n = 3; Benign, n = 6; Borderline, n = 3; Malignant, n = 4). d The expression of total STAT1, pSTAT1-Y701, and pSTAT1-S727 detected by immunoblotting. Representative images are shown. STAT1α, 91 kDa; STAT1β, 84 kDa. e Densitometric analysis of the gels in (d) (Normal, n = 5; Benign, n = 6; Borderline, n = 3; Malignant, n = 6). *, P < 0.05 compared to normal tissue
Fig. 2
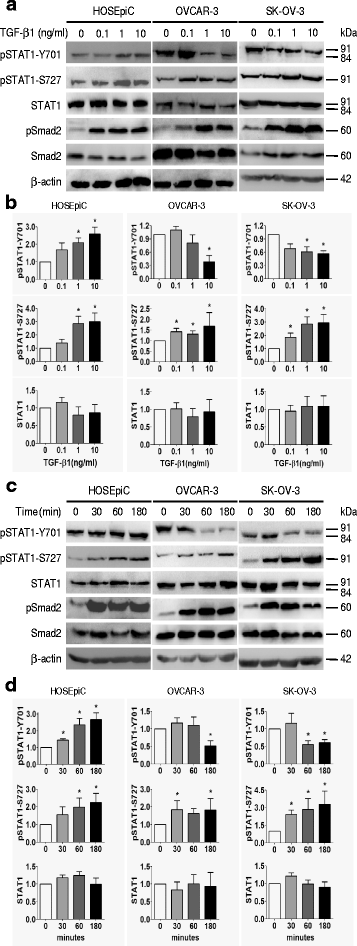
TGF-β1 regulates the phosphorylation of STAT1 in ovarian surface epithelial cells. Dose-dependent (a, b) and time-course (c, d) studies of the effect of TGF-β1 on STAT1 phosphorylation (pSTAT1) are shown. a Immunoblotting after cells treated with TGF-β1 (0, 0.1, 1, and 10 ng/ml) for 24 h. b Densitometry analysis of the gels in (a). c Immunoblotting after cells treated with TGF-β1 (10 ng/ml) for 30, 60, and 180 min. d Densitometric analysis of the gels in (c). The phosphorylation of Smad2 (pSamd2) is increased after TGF-β1 treatment, indicating the responsiveness of cells to TGF-β1. Data are represented as the mean ± SEM of the ratio of pSTAT1/total STAT1 and total STAT1/β-actin. n = 3 independent experiments; *, P < 0.05 compared to untreated cells
Fig. 3
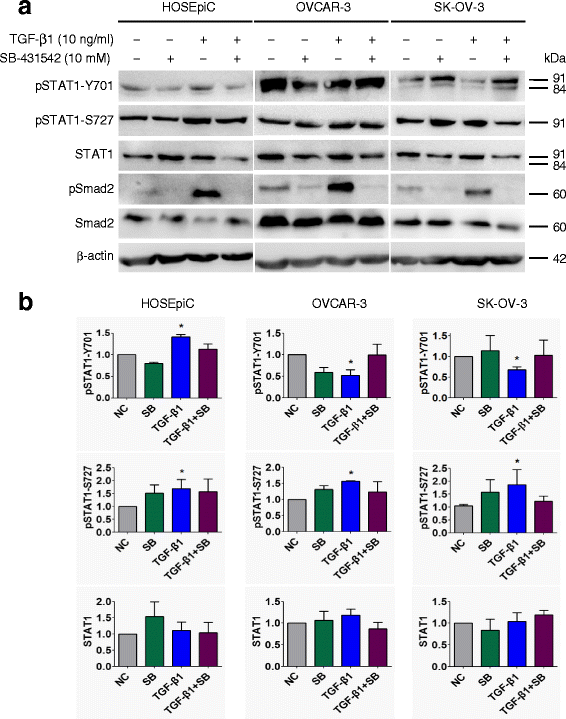
Inhibition of TGF-β receptor kinase blocks the effect of TGF-β on STAT1 phosphorylation in ovarian surface epithelial cells. Cells were pretreated with 10 μM TβRI inhibitor SB-431542 for 30 min, followed by 10 ng/ml of TGF-β1 treatment for 24 h. a Immunoblotting after cells treated with SB-431542 and/or TGF-β1. b Densitometric analysis of the gels in (a). The responsiveness of cells to TGF-β1 was confirmed by the detection of pSmad2. Data are represented as the mean ± SEM of the ratio of pSTAT1 over total STAT1 or total STAT1 over β-actin. NC, non-treated control; SB, SB-431542; n = 3 independent experiments; *, P < 0.05 compared to NC
Fig. 4
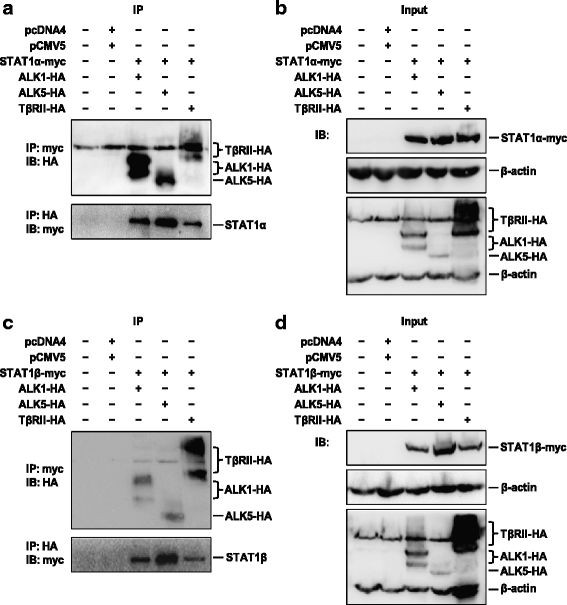
STAT1 interacts with the TGF-β1 receptor. HEK-293 T cells were transiently co-transfected with STAT1α-myc or STAT1β-myc and ALK1-HA, ALK5-HA or TβRII-HA plasmids and incubated for 48 h. a Interaction of STAT1α with receptors detected by immunoprecipitation (IP) with anti-myc antibody for STAT1α, followed by immunoblotting (IB) with anti-HA antibody for receptors (upper panel), or IP with anti-HA antibody for receptors, followed by IB with anti-myc antibody for STAT1α (bottom panel). b IB using antibodies specific to β-actin, myc for STAT1α, and HA for receptors. c Interaction of STAT1β with receptors detected by IP with anti-myc antibody for STAT1β, followed by IB with anti-HA antibody for receptors (upper panel), or vice versa (bottom panel). d IB using antibodies specific to β-actin, myc for STAT1β, and HA for receptors. pcDNA4 and pCMV5 are two empty vectors used as negative controls. Each experiment is repeated at least once. Representative images are shown. STAT1α-myc, 93 kDa; STAT1β-myc, 86 kDa; ALK1-HA, 58–69 kDa; ALK5-HA, 53 kDa; TβRII-HA, 71–80 kDa
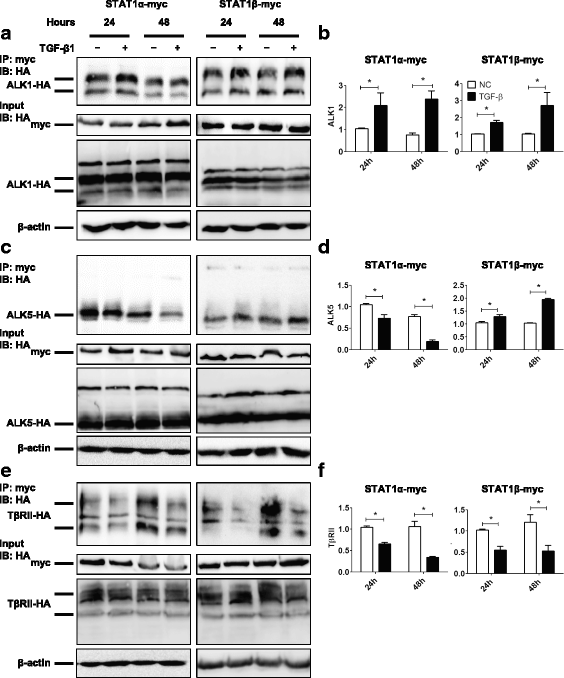
Fig. 5
TGF-β1 regulates the interaction of STAT1α/STAT1β with ALK1/ALK5/TβRII. HEK-293 T cells were transiently co-transfected with STAT1α-myc or STAT1β-myc and ALK1-HA, ALK5-HA or TβRII-HA plasmids in the absence or presence of TGF-β1 (10 ng/ml) for 24 and 48 h. a Immunoprecipitation (IP) with anti-myc antibody for STAT1α-myc or STAT1β-myc and immunoblotting (IB) with anti-HA antibody for ALK1-HA. Cell lysates of input samples were used for detecting the expression of STAT1-myc, ALK1-HA, and β-actin. b Densitometric and semi-quantitative analyses of the gels of IP in (a). c IP with anti-myc antibody for STAT1α-myc or STAT1β-myc and IB with anti-HA antibody for ALK5-HA. Cell lysates of input samples were used for detecting the expression of STAT1-myc, ALK5-HA, and β-actin. d Densitometric and semi-quantitative analyses of the gels of IP in (c). e IP with anti-myc antibody for STAT1α-myc or STAT1β-myc and IB with anti-HA antibody for TβRII-HA. Cell lysates of input samples were used for detecting the expression of STAT1-myc, TβRII-HA, and β-actin. f Densitometric and semi-quantitative analyses of the gels of IP in (e). n = 3 independent experiments; *, P < 0.05
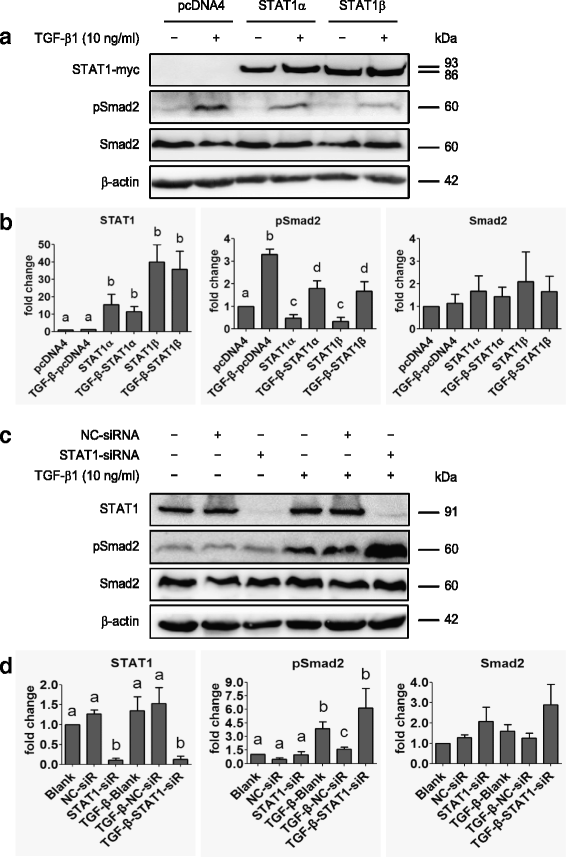
Fig. 6
STAT1 affects Smad2 phosphorylation. a Immunoblotting after SK-OV-3 cells transiently transfected with pcDNA4, STAT1α or STAT1β plasmid in the absence or presence of TGF-β1 (10 ng/ml) for 24 h. b Densitometric analysis of the gels in (a). c Immunoblotting of STAT1 and Smad2 in STAT1-knockdown SK-OV-3 cells in the absence or presence of TGF-β1 (10 ng/ml) for 24 h. d Densitometric analysis of the gels in (c). Data are represented as the mean ± SEM of the ratio of pSmad2/total Smad2, total Smad2/β-actin, and total STAT1/β-actin. Different superscript denotes statistically significant difference (P < 0.05; n = 3 independent experiments)
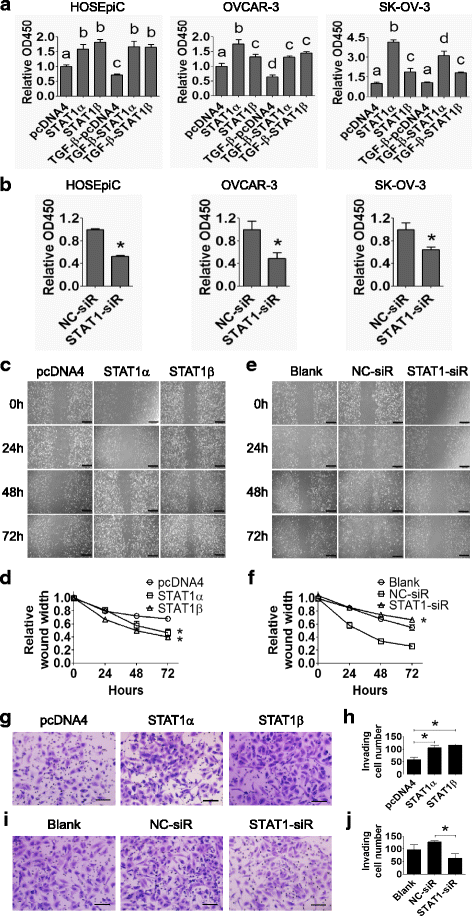
Fig. 7
STAT1 increases ovarian surface epithelial cell proliferation, migration, and invasion. a HOSEpiC, OVCAR-3, and SK-OV-3 cells were transiently transfected with STAT1α or STAT1β plasmid in the absence or presence of TGF-β1 (10 ng/ml) for 48 h. Cell proliferation was measured by the WST-1 assay. pcDNA4 was used as negative control. Different superscript denotes statistically significant difference (P < 0.05; n = 3 independent experiments). b HOSEpiC, OVCAR-3, and SK-OV-3 cells were transiently transfected with STAT1-siRNA or non-specific control (NC)-siRNA. Cell proliferation was measured by the WST-1 assay at 48 h post-transfection (*, P < 0.05; n = 3 independent experiments). c Wound healing assay in SK-OV-3 cells after transiently transfecting with STAT1α or STAT1β plasmid for 24, 48, and 72 h. d Quantitative analysis of the wound width in (c). e Wound healing assay in SK-OV-3 cells after transiently transfecting with STAT1-siRNA (STAT1-siR) for 24, 48, and 72 h. f Quantitative analysis of the wound width in (e). STAT1 promotes the migration of SK-OV-3 cells. Original magnification, × 200; scale bars, 500 μm. g Invasion assay of SK-OV-3 cells after transiently transfecting with STAT1α or STAT1β plasmid for 48 h. h Quantitative analysis of invaded cells in (g). i Invasion assay of SK-OV-3 cells after transiently transfecting with STAT1-siRNA or NC-siRNA for 48 h. j Quantification analysis of invaded cells in (i). Invaded cells were counted from three random fields. STAT1 promotes the invasion of SK-OV-3 cells. Original magnification, × 200; scale bar, 500 μm. Data are presented as the mean ± standard deviation (SD). *, P < 0.05 compared to control
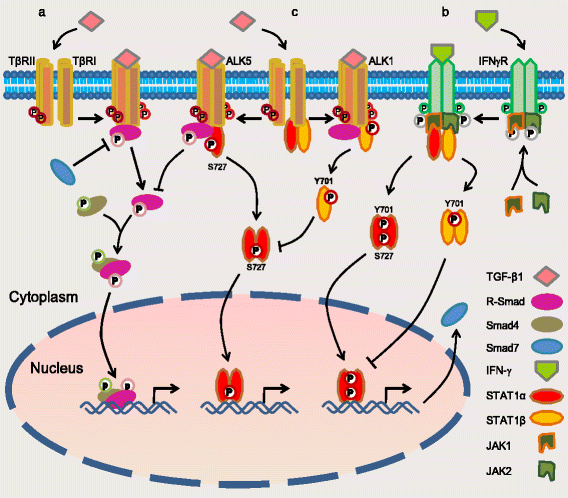
Fig. 8
A schematic of the model of crosstalk between STAT1 and TGF-β signaling pathways. a The canonical signaling pathway of TGF-β: upon TGF-β1 binding to type II receptor (TβRII), the activated TβRII recruits and activates type I receptor (TβRI: ALK5 in most type of cells or ALK1 in endothelial cells). The activated receptor kinases then phosphorylate R-Smads such as Smad2/3 (by ALK5) and Smad1/5/8 (by ALK1). Activated R-Smads form the complex with Smad4 and translocate to the nucleus where they regulate target genes [12, 36]. b The canonical signaling pathway of STAT1: upon IFN-γ binding to its receptor, JAKs are phosphorylated and activated each other. The activated JAKs along with the intracellular tail of receptor recruit and activate STAT1 by the phosphorylation of STAT1 on Y701 and/or S727, which promotes them to dimerize and enter the nucleus where they regulate gene expression [7, 37], e.g. gene Smad7. Protein Smad7 prevents R-Smad interaction with TGF-β receptor [8]. Overexpression of STAT1β inhibits STAT1α activity to maintain the balance between two isoforms [26]. c The crosstalk of the STAT1 and TGF-β signaling pathways: STAT1 constitutively interacts with TβRII/TβRI (either ALK5 or ALK1 based on cell type). Upon TGF-β1 binding to TβRII/ALK5, the receptor-complex increases the phosphorylation of STAT1 on S727 (the active form of STAT1α). The activated STAT1 then dissociates itself from TβRII/ALK5 complex and executes its mission of transcription factor. On the other hand, STAT1 protein is increased in ovarian cancer cells and binds to TGF-β receptors. Overexpressed STAT1 suppresses TGF-β-induced Smad2 phosphorylation and blocks, at least in part, the TGF-β signaling pathway (current work)
Similar articles
- Pyridoxine 5'-phosphate oxidase is a novel therapeutic target and regulated by the TGF-β signalling pathway in epithelial ovarian cancer.
Zhang L, Zhou D, Guan W, Ren W, Sun W, Shi J, Lin Q, Zhang J, Qiao T, Ye Y, Wu Y, Zhang Y, Zuo X, Connor KL, Xu G. Zhang L, et al. Cell Death Dis. 2017 Dec 13;8(12):3214. doi: 10.1038/s41419-017-0050-3. Cell Death Dis. 2017. PMID: 29238081 Free PMC article. - Cytidine monophosphate kinase is inhibited by the TGF-β signalling pathway through the upregulation of miR-130b-3p in human epithelial ovarian cancer.
Zhou D, Zhang L, Sun W, Guan W, Lin Q, Ren W, Zhang J, Xu G. Zhou D, et al. Cell Signal. 2017 Jul;35:197-207. doi: 10.1016/j.cellsig.2017.04.009. Epub 2017 Apr 14. Cell Signal. 2017. PMID: 28414100 - Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-Smad 3 pathway.
Yoo YA, Kang MH, Kim JS, Oh SC. Yoo YA, et al. Carcinogenesis. 2008 Mar;29(3):480-90. doi: 10.1093/carcin/bgm281. Epub 2008 Jan 3. Carcinogenesis. 2008. PMID: 18174246 - Proteinase-Activated Receptor 2 May Drive Cancer Progression by Facilitating TGF-β Signaling.
Ungefroren H, Witte D, Rauch BH, Settmacher U, Lehnert H, Gieseler F, Kaufmann R. Ungefroren H, et al. Int J Mol Sci. 2017 Nov 22;18(11):2494. doi: 10.3390/ijms18112494. Int J Mol Sci. 2017. PMID: 29165389 Free PMC article. Review. - 4-hydroxynonenal and transforming growth factor-beta1 expression in colon cancer.
Zanetti D, Poli G, Vizio B, Zingaro B, Chiarpotto E, Biasi F. Zanetti D, et al. Mol Aspects Med. 2003 Aug-Oct;24(4-5):273-80. doi: 10.1016/s0098-2997(03)00022-0. Mol Aspects Med. 2003. PMID: 12893005 Review.
Cited by
- STAT1 suppresses the transcriptional activity of TRIM21 in gastric cancer.
Huo C, Gu Y, Wang D, Zhang X, Tang F, Zhao B, Liu T, He W, Li Y. Huo C, et al. J Cancer Res Clin Oncol. 2023 Nov;149(17):15335-15348. doi: 10.1007/s00432-023-05307-8. Epub 2023 Aug 28. J Cancer Res Clin Oncol. 2023. PMID: 37639009 - Integrated analysis of transcriptomic and metabolomic data demonstrates the significant role of pyruvate carboxylase in the progression of ovarian cancer.
Shang H, Zheng J, Tong J. Shang H, et al. Aging (Albany NY). 2020 Nov 7;12(21):21874-21889. doi: 10.18632/aging.104004. Epub 2020 Nov 7. Aging (Albany NY). 2020. PMID: 33177242 Free PMC article. - An L-type calcium channel blocker nimodipine exerts anti-fibrotic effects by attenuating TGF-β1 induced calcium response in an in vitro model of thyroid eye disease.
Chen Q, Pan Y, Hu Y, Chen G, Chen X, Xie Y, Wang M, Li Z, Huang J, Shi Y, Huang H, Zhang T, Wang M, Zeng P, Wang S, Chen R, Zheng Y, Zhong L, Yang H, Liang D. Chen Q, et al. Eye Vis (Lond). 2024 Sep 6;11(1):37. doi: 10.1186/s40662-024-00401-5. Eye Vis (Lond). 2024. PMID: 39237996 Free PMC article. - Transforming growth factor β1 promotes fibroblast-like synoviocytes migration and invasion via TGF-β1/Smad signaling in rheumatoid arthritis.
Zhu D, Zhao J, Lou A, Huang Q, OuYang Q, Zhu J, Fan M, He Y, Ren H, Yang M. Zhu D, et al. Mol Cell Biochem. 2019 Sep;459(1-2):141-150. doi: 10.1007/s11010-019-03557-0. Epub 2019 Jul 11. Mol Cell Biochem. 2019. PMID: 31297660 - Inflammation, Fibrosis and Cancer: Mechanisms, Therapeutic Options and Challenges.
Wu B, Sodji QH, Oyelere AK. Wu B, et al. Cancers (Basel). 2022 Jan 22;14(3):552. doi: 10.3390/cancers14030552. Cancers (Basel). 2022. PMID: 35158821 Free PMC article. Review.
References
- Najjar I, Schischmanoff PO, Baran-Marszak F, Deglesne PA, Youlyouz-Marfak I, Pampin M, Feuillard J, Bornkamm GW, Chelbi-Alix MK, Fagard R. Novel function of STAT1beta in B cells: induction of cell death by a mechanism different from that of STAT1alpha. J Leukoc Biol. 2008;84:1604–1612. doi: 10.1189/jlb.0508287. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- 81272880/National Natural Science Foundation of China
- 17ZR1404100/Natural Science Foundation of Shanghai
- 201640287/Shanghai Municipal Commission of Health and Family Planning
- 124119b1300/Science and Technology Commission of Shanghai Municipality
- JSKJ-KTMS-2015-01/Committee of Science and Technology of Jinshan District
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous