A Liquid to Solid Phase Transition Underlying Pathological Huntingtin Exon1 Aggregation - PubMed (original) (raw)
A Liquid to Solid Phase Transition Underlying Pathological Huntingtin Exon1 Aggregation
Thomas R Peskett et al. Mol Cell. 2018.
Abstract
Huntington's disease is caused by an abnormally long polyglutamine tract in the huntingtin protein. This leads to the generation and deposition of N-terminal exon1 fragments of the protein in intracellular aggregates. We combined electron tomography and quantitative fluorescence microscopy to analyze the structural and material properties of huntingtin exon1 assemblies in mammalian cells, in yeast, and in vitro. We found that huntingtin exon1 proteins can form reversible liquid-like assemblies, a process driven by huntingtin's polyQ tract and proline-rich region. In cells and in vitro, the liquid-like assemblies converted to solid-like assemblies with a fibrillar structure. Intracellular phase transitions of polyglutamine proteins could play a role in initiating irreversible pathological aggregation.
Keywords: aggregation; electron tomography; fluorescence microscopy; huntingtin exon1; phase transition; polyQ.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures
Graphical abstract
Figure 1
Aggregation of HTTex1 Proteins Can Involve a Conversion between Distinct Macroscopic Assemblies (A) Domain organization of HTTex1 constructs in this study. (B) Representative confocal maximum intensity projections of bright and dim 43QP-GFP assemblies. Scale bar, 10 μm. (C and D) Time-lapse fluorescence microscopy of 43QP-GFP aggregation without (C), and with (D), a visible intermediate dim assembly. Orange arrows: bright assembly formation. Blue asterisk: coalescence of dim assemblies. Scale bar, 10 μm. (E) Quantification of aggregation events occurring without (orange) and with (orange/blue) visible intermediate dim assemblies. n > 92 aggregation events per construct from three independent experiments. p = 0.0003, chi-square. (F) FRAP experiment showing high HTTex1 mobility in dim assemblies but not in bright assemblies. Scale bar, 3 μm. (G) Averaged FRAP recovery curves. Shaded areas represent 95% confidence interval (CI). Dim assemblies estimated mobile fraction = 84%, 95% CI: 83%–85%, n = 20; bright assemblies estimated mobile fraction = 10%, 95% CI: 10%–11%, n = 20. See also Table S1. (H) EM projection image of a 43QP-GFP bright assembly (orange dashes). Higher-magnification image (inset) shows a network of fibrillar structures. (I) 43QP-GFP bright assembly (orange) and dim assemblies (blue). Higher-magnification image (inset) shows fibrillar structures emanating from the dim assemblies. Low-magnification scale bar, 5 μm; high-magnification scale bar, 500 nm.
Figure 2
Probing HTTex1 Assembly States in Yeast (A) Widefield microscopy of 43QP-GFP assemblies formed in [RNQ+] cells (green arrows), and [rnq−] cells (blue and orange arrows, showing dim and bright assemblies, respectively). Scale bar, 5 μm. (B) Averaged FRAP recovery curves. Shaded areas: 95% confidence intervals. See also Table S1. (C) Estimated mobile fractions of assemblies based on (B). Bright [rnq−] versus dim [rnq−] p = 6.3 × 10–13; bright [rnq−] versus [RNQ+] p = 0.003; dim [rnq−] versus [RNQ+] p = 3.2 × 10–12; Welch’s two-sample t test. (D) Photo bleaching of a dim assembly (red outline) in a yeast cell (gray outline) containing a second, coexisting dim assembly (purple outline). Scale bar, 3 μm. (E) Quantification of data in (D). Solid lines show mean fluorescence intensities in the corresponding colored regions in (D). Dotted lines highlight initial fluorescence levels in the corresponding colored regions. See also Table S2. (F) Intensity ratios of assemblies. Bright [rnq−] versus dim [rnq−] p = 0.0009; bright [rnq−] versus [RNQ+] p = 0.16; dim [rnq−] versus [RNQ+] p = 0.0019; Welch’s two sample t test. Black dots represent individual data points that fell outside the whiskers of the boxplots. (G) Circularity ratios of assemblies. Bright [rnq−] versus dim [rnq−] p = 0.0001; bright [rnq−] versus [RNQ+] p = 0.04; dim [rnq−] versus [RNQ+] p = 0.0006; Welch’s two-sample t test. (H) Plot of intensity ratios versus circularity ratios.
Figure 3
Dim Assemblies Display Liquid-like Properties in Cells (A) Addition of 1,6-hexanediol to digitonin-permeabilised cells can discriminate between liquid-like assemblies, which dissolve, and solid-like assemblies, which do not (Kroschwald et al., 2015). (B) Kymographs showing the effect of hexanediol addition on fluorescence intensities of HTTex1 assemblies in digitonin-permeabilized yeast cells. Hexanediol was added at time = 0 s. (C) Quantification of fluorescence intensities in kymographs in (B). (D) Snapshots and kymograph of hexanediol addition (first dotted white line) to a yeast cell containing dim assemblies, followed by hexanediol removal (second dotted white line). The red line indicates the slice through which the kymograph was plotted. Blue arrow highlights coalescence. Images were linearly scaled to increase the visibility of the merging assemblies, saturating some of the pixels in the early frames. Note that dim assemblies do not sequester all the cytoplasmic fluorescence, which is visible throughout the experiment. Scale bar, 3 μm. Related to Video S4.
Figure 4
Liquid-like and Solid-like HTTex1 Assemblies Have Different Nanostructures Electron tomography of yeast cells expressing 43Q-GFP and 43QP-GFP. Panels show slices through tomograms of the HTTex1 assemblies. Colors indicate the type of assembly and prion status of the cells: orange, [rnq−] SAs; blue, [rnq−] LAs; green, [RNQ+] SAs. (A) 43Q-GFP SA in a [rnq−] cell, showing a fibrillar nanostructure. (B) 43Q-GFP LA in a [rnq−] cell, with a characteristic “smooth” appearance. (C) 43Q-GFP SA in a [RNQ+] cell. (D) 43QP-GFP SA in a [rnq−] cell. (E) 43QP-GFP LA in a [rnq−] cell. (F) 43QP-GFP SA in a [RNQ+] cell. Scale bar, 200 nm.
Figure 5
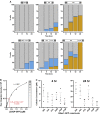
HTTex1 Sequence Affects Assembly Formation (A) Yeast cells in the [rnq−] background expressing HTTex1 constructs with different polyQ lengths, with or without the P-rich region, were imaged by widefield fluorescence microscopy at specific time points after induction of HTTex1 expression. The percentage of cells containing an SA (orange), an LA (blue), or diffuse fluorescence (gray) was determined at each time point by manual counting. n = 207–259 cells per construct. (B) Quantitative dot blots calibrated with purified 25QP-GFP were used to determine HTTex1 concentration in cellular lysates (red). (C) Cellular concentrations of HTTex1 constructs at 4 and 24 hr. Plots show individual experiments (black) and mean ± SEM (gray).
Figure 6
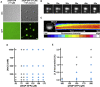
HTTex1 Forms Liquid-like Assemblies by Liquid-Liquid Phase Separation (A) Liquid-liquid phase separation of 25QP-GFP induced by molecular crowding. Scale bar, 5 μm. (B) Fusion of 25QP-GFP droplets. Scale bar, 2 μm. See also Video S5. (C) Half-bleach (Brangwynne et al., 2009) of a 25QP-GFP droplet (white dashed outline). Kymograph shows redistribution of 25QP-GFP after the bleach. Scale bar, 1 μm. (D and E) Intermolecular interactions of 25QP-GFP in droplets. (D) Droplet formation at different salt and protein concentrations. The phase diagram indicates conditions where 25QP-GFP forms droplets (blue dots) and where it does not (gray dots). (E) Phase diagram showing the effect of 1,6-hexanediol concentrations on droplet formation. All experiments were carried out in the presence of 10% dextran as a crowding agent, and each condition was assessed at least twice.
Figure 7
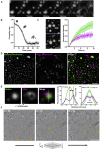
Liquid-like HTTex1 Assemblies Convert into Solid-like Assemblies (A) Liquid-like droplets gradually convert into solid-like structures. Scale bar, 2 μm. (B) Quantification of the mean circularity of droplets during the conversion shown in (A). (C) FRAP of partially converted droplets comparing recovery of spike features (magenta) and droplet centers (green). Shaded region of graph represents 95% confidence interval. Scale bar, 2 μm. See also Table S1. (D) Droplets before (green) and after (magenta) hexanediol addition. Scale bar, 5 μm. (E) Quantification of early stages of droplet conversion (related to Figure 7D). Radial shift is the distance between intensity maxima in droplet linescans before (green) and after (magenta) hexanediol. n = 20 similarly sized droplets. Shaded regions: 95% confidence intervals. Scale bar, 0.5 μm. (F) Slices through a tomogram of a yeast cell containing a 43Q-GFP liquid-like assembly (blue dotted line) containing isolated fibrillar structures (orange arrows). Scale bar, 200 nm.
Comment in
- Forms and Phases in Huntingtin Protein Aggregation.
Elbaum M. Elbaum M. Mol Cell. 2018 May 17;70(4):567-568. doi: 10.1016/j.molcel.2018.05.004. Mol Cell. 2018. PMID: 29775574
Similar articles
- Fibril polymorphism affects immobilized non-amyloid flanking domains of huntingtin exon1 rather than its polyglutamine core.
Lin HK, Boatz JC, Krabbendam IE, Kodali R, Hou Z, Wetzel R, Dolga AM, Poirier MA, van der Wel PCA. Lin HK, et al. Nat Commun. 2017 May 24;8:15462. doi: 10.1038/ncomms15462. Nat Commun. 2017. PMID: 28537272 Free PMC article. - Binding, internalization and fate of Huntingtin Exon1 fibrillar assemblies in mitotic and nonmitotic neuroblastoma cells.
Ruiz-Arlandis G, Pieri L, Bousset L, Melki R. Ruiz-Arlandis G, et al. Neuropathol Appl Neurobiol. 2016 Feb;42(2):137-52. doi: 10.1111/nan.12258. Epub 2015 Jul 20. Neuropathol Appl Neurobiol. 2016. PMID: 26111612 - Autophagy preferentially degrades non-fibrillar polyQ aggregates.
Zhao DY, Bäuerlein FJB, Saha I, Hartl FU, Baumeister W, Wilfling F. Zhao DY, et al. Mol Cell. 2024 May 16;84(10):1980-1994.e8. doi: 10.1016/j.molcel.2024.04.018. Mol Cell. 2024. PMID: 38759629 - Visualization of prion-like transfer in Huntington's disease models.
Jansen AH, Batenburg KL, Pecho-Vrieseling E, Reits EA. Jansen AH, et al. Biochim Biophys Acta Mol Basis Dis. 2017 Mar;1863(3):793-800. doi: 10.1016/j.bbadis.2016.12.015. Epub 2016 Dec 29. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28040507 Review. - Spontaneous self-assembly of pathogenic huntingtin exon 1 protein into amyloid structures.
Trepte P, Strempel N, Wanker EE. Trepte P, et al. Essays Biochem. 2014;56:167-80. doi: 10.1042/bse0560167. Essays Biochem. 2014. PMID: 25131594 Review.
Cited by
- Macromolecular Crowding, Phase Separation, and Homeostasis in the Orchestration of Bacterial Cellular Functions.
Monterroso B, Margolin W, Boersma AJ, Rivas G, Poolman B, Zorrilla S. Monterroso B, et al. Chem Rev. 2024 Feb 28;124(4):1899-1949. doi: 10.1021/acs.chemrev.3c00622. Epub 2024 Feb 8. Chem Rev. 2024. PMID: 38331392 Free PMC article. Review. - A modular tool to query and inducibly disrupt biomolecular condensates.
Hernández-Candia CN, Pearce S, Tucker CL. Hernández-Candia CN, et al. Nat Commun. 2021 Mar 22;12(1):1809. doi: 10.1038/s41467-021-22096-1. Nat Commun. 2021. PMID: 33753744 Free PMC article. - Nascent mutant Huntingtin exon 1 chains do not stall on ribosomes during translation but aggregates do recruit machinery involved in ribosome quality control and RNA.
Ormsby AR, Cox D, Daly J, Priest D, Hinde E, Hatters DM. Ormsby AR, et al. PLoS One. 2020 Jul 31;15(7):e0233583. doi: 10.1371/journal.pone.0233583. eCollection 2020. PLoS One. 2020. PMID: 32735619 Free PMC article. - Nucleolar Sequestration: Remodeling Nucleoli Into Amyloid Bodies.
Wang M, Bokros M, Theodoridis PR, Lee S. Wang M, et al. Front Genet. 2019 Nov 21;10:1179. doi: 10.3389/fgene.2019.01179. eCollection 2019. Front Genet. 2019. PMID: 31824572 Free PMC article. - Biomolecular condensate phase diagrams with a combinatorial microdroplet platform.
Arter WE, Qi R, Erkamp NA, Krainer G, Didi K, Welsh TJ, Acker J, Nixon-Abell J, Qamar S, Guillén-Boixet J, Franzmann TM, Kuster D, Hyman AA, Borodavka A, George-Hyslop PS, Alberti S, Knowles TPJ. Arter WE, et al. Nat Commun. 2022 Dec 21;13(1):7845. doi: 10.1038/s41467-022-35265-7. Nat Commun. 2022. PMID: 36543777 Free PMC article.
References
- Abadi M., Agarwal A., Barham P., Brevdo E., Chen Z., Citro C., Corrado G.S., Davis A., Dean J., Devin M. OSDI’16 Proceedings of the 12th USENIX Conference on Operating Systems Design and Implementation. 2016. TensorFlow: Large-Scale Machine Learning on Heterogeneous Distributed Systems; pp. 265–283.
- Arrasate M., Mitra S., Schweitzer E.S., Segal M.R., Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431:805–810. - PubMed
- Bates G.P., Dorsey R., Gusella J.F., Hayden M.R., Kay C., Leavitt B.R., Nance M., Ross C.A., Scahill R.I., Wetzel R. Huntington disease. Nat. Rev. Dis. Primers. 2015;1:15005. - PubMed
- Bäuerlein F.J.B., Saha I., Mishra A., Kalemanov M., Martínez-Sánchez A., Klein R., Dudanova I., Hipp M.S., Hartl F.U., Baumeister W., Fernández-Busnadiego R. In situ architecture and cellular interactions of PolyQ inclusions. Cell. 2017;171:179–187.e10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 102401/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- 101488/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- 106249/Z/14/Z/WT_/Wellcome Trust/United Kingdom
- 079605/Z/06/Z/WT_/Wellcome Trust/United Kingdom
- 101149/Z/13/A/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- MR/K015826/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous