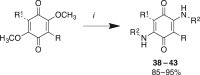A focused library synthesis and cytotoxicity of quinones derived from the natural product bolinaquinone - PubMed (original) (raw)
A focused library synthesis and cytotoxicity of quinones derived from the natural product bolinaquinone
Azadeh Ghods et al. R Soc Open Sci. 2018.
Abstract
Bolinaquinone is a natural product that is a structurally complex, cytotoxic sesquiterpene quinone. A scaffold simplification and focused library approach using a microwave-assisted Suzuki coupling gave 32 bolinaquinone analogues with good-to-excellent cytotoxicity profiles. Mono-arylbenzoquinones, Library A, were preferentially toxic towards BE2-C (neuroblastoma) cells with growth inhibition (GI50) values of 4-12 µM; only the 3,4-dimethoxyphenyl 23 and 3-biphenyl 28 variants were broad-spectrum active-HT29 (colon carcinoma), U87 and SJ-G2 (glioblastoma), MCF-7 (breast carcinoma), A2780 (ovarian carcinoma), H460 (lung carcinoma), A431 (skin carcinoma), Du145 (prostate carcinoma), BE2-C (neuroblastoma), MIA (pancreatic carcinoma) and SMA (spontaneous murine astrocytoma). Library B with a second aryl moiety exhibited broad-spectrum cytotoxicity with MCF-7 cells' GI50 values of 5.6 ± 0.7 and 5.1 ± 0.5 µM for 2,5-dimethoxy-3-(naphthalene-1-yl)-6-(naphthalene-3-yl) 33 and 2,5-dimethoxy-3-(biaryl-2-yl)-6-(naphthalene-3-yl) 36, respectively. Similar potencies were also noted with 2,5-dimethoxy-3,6-diphenyl 30 against A2780 (GI50 = 5.9 ± 0.0 µM) and with 2,5-dimethoxy-3-(biaryl-3-yl)-6-(naphthalene-3-yl) 37 against HT29 (GI50 = 5.4 ± 0.4 µM), while the 3,4-dimethoxy mono-aryl analogue 23 exhibited good levels of activity against A2780 (GI50 = 3.8 ± 0.75 µM), the neuroblastoma cell line BE2-C (GI50 = 3 ± 0.35 µM) and SMA (GI50 = 3.9 ± 0.54 µM). Introduction of the amino-substituted Library C gave 2-(naphthalen-1-yl)-5-(naphthalen-3-yl)-3,6-bis(propylamino) 43, with excellent activity against HT29 (0.08 ± 0.0 µM), MCF-7 (0.17 ± 0.1 µM), A2780 (0.14 ± 0.1 µM), A431 (0.11 ± 0.0 µM), Du145 (0.16 ± 0.1 µM), BE2-C (0.08 ± 0.0 µM) and MIA (0.1 ± 0.0 µM).
Keywords: Suzuki coupling; bolinaquinone; cytotoxicity; microwave-assisted synthesis.
Conflict of interest statement
We declare we have no competing interests.
Figures
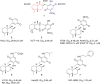
Figure 1.
The chemical structures of selected sesquiterpene quinones: bolinaquinone (1), avarone (2), mamanuthaquinone (3), epi-ilimaquinone (4), nakijiquinone A (5), dysidaminone E-5 (6) and 18-phenethylaminoavarone (7), and their reported cytotoxicity towards different cancer cell lines.
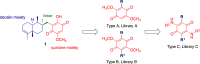
Figure 2.
The proposed structural simplification of the lead, bolinaquinone 1, to afford three bolinaquinone libraries: A, B and C.
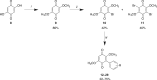
Scheme 1.
Reagents and conditions: (i) MeOH, H2SO4, 25°C, 6 h; (ii) NBS, DMF, 60°C (then 25°C), 5–8 h; (iii) arylboronic acid (Ar-B(OH)2), Pd(dppf)Cl2, K2CO3, dioxane, µW, 120°C, 20 min, or arylboronic acid (R-B(OH)2), Pd(dppf)Cl2, K2CO3, toluene, reflux, 72 h.
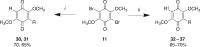
Scheme 2.
Reagents and conditions: (i) 2 equivalents of arylboronic acid (R-B(OH)2), Pd(dppf)Cl2, K2CO3, dioxane, μW, 120°C, 20 min; (ii) 1 equivalent of arylboronic acid (R-B(OH)2), Pd(dppf)Cl2, K2CO3, dioxane, μW, 120°C, 20 min, then 1 equivalent of arylboronic acid (R'-B(OH)2), 120°C, 20 min.
Scheme 3.
Reagent and conditions: (i) _n_-propyl amine or _N,N_-dimethylpropane-1,3-diamine, MeOH, 25°C, 1 h.
Similar articles
- Dichlorophenylacrylonitriles as AhR Ligands That Display Selective Breast Cancer Cytotoxicity in vitro.
Baker JR, Gilbert J, Paula S, Zhu X, Sakoff JA, McCluskey A. Baker JR, et al. ChemMedChem. 2018 Jul 18;13(14):1447-1458. doi: 10.1002/cmdc.201800256. Epub 2018 Jul 2. ChemMedChem. 2018. PMID: 29771007 - Synthesis and Cytotoxicity of Octahydroepoxyisoindole-7-carboxylic Acids and Norcantharidin-Amide Hybrids as Norcantharidin Analogues.
Hizartzidis L, Gilbert J, Gordon CP, Sakoff JA, McCluskey A. Hizartzidis L, et al. ChemMedChem. 2019 Jun 18;14(12):1152-1161. doi: 10.1002/cmdc.201900180. Epub 2019 May 14. ChemMedChem. 2019. PMID: 30938091 - 3,5-Bis(trifluoromethyl)phenylsulfonamides, a novel pancreatic cancer active lead. Investigation of the terminal aromatic moiety.
Sun J, Ambrus JI, Baker JR, Russell CC, Cossar PJ, Sakoff JA, Scarlett CJ, McCluskey A. Sun J, et al. Bioorg Med Chem Lett. 2022 Apr 1;61:128591. doi: 10.1016/j.bmcl.2022.128591. Epub 2022 Jan 31. Bioorg Med Chem Lett. 2022. PMID: 35114371 - Cytotoxic compounds from Laurencia pacifica.
Zaleta-Pinet DA, Holland IP, Muñoz-Ochoa M, Murillo-Alvarez JI, Sakoff JA, van Altena IA, McCluskey A. Zaleta-Pinet DA, et al. Org Med Chem Lett. 2014 Dec;4(1):8. doi: 10.1186/s13588-014-0008-8. Epub 2014 Sep 20. Org Med Chem Lett. 2014. PMID: 26548986 Free PMC article. - Pyrimidyn-Based Dynamin Inhibitors as Novel Cytotoxic Agents.
Odell LR, Chau N, Russell CC, Young KA, Gilbert J, Robinson PJ, Sakoff JA, McCluskey A. Odell LR, et al. ChemMedChem. 2022 Jan 5;17(1):e202100560. doi: 10.1002/cmdc.202100560. Epub 2021 Nov 10. ChemMedChem. 2022. PMID: 34590434
Cited by
- The Chemical Inhibitors of Endocytosis: From Mechanisms to Potential Clinical Applications.
Szewczyk-Roszczenko OK, Roszczenko P, Shmakova A, Finiuk N, Holota S, Lesyk R, Bielawska A, Vassetzky Y, Bielawski K. Szewczyk-Roszczenko OK, et al. Cells. 2023 Sep 19;12(18):2312. doi: 10.3390/cells12182312. Cells. 2023. PMID: 37759535 Free PMC article. Review. - 2,3-Dihydroquinazolin-4(1_H_)-ones and quinazolin-4(3_H_)-ones as broad-spectrum cytotoxic agents and their impact on tubulin polymerisation.
O'Brien NS, Gilbert J, McCluskey A, Sakoff JA. O'Brien NS, et al. RSC Med Chem. 2024 Mar 6;15(5):1686-1708. doi: 10.1039/d3md00600j. eCollection 2024 May 22. RSC Med Chem. 2024. PMID: 38784470 - α-Mangostin Promotes In Vitro and In Vivo Degradation of Androgen Receptor and AR-V7 Splice Variant in Prostate Cancer Cells.
Nauman MC, Won JH, Petiwala SM, Vemu B, Lee H, Sverdlov M, Johnson JJ. Nauman MC, et al. Cancers (Basel). 2023 Apr 1;15(7):2118. doi: 10.3390/cancers15072118. Cancers (Basel). 2023. PMID: 37046780 Free PMC article. - Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review.
References
- AIHW & AACR. 2012. Cancer in Australia: an overview. 2012 Cancer series no. 74. Cat. no. CAN 70. Canberra, Australia: AIHW.
- Joshi S, et al. 2010. The dynamin inhibitors MiTMAB and OcTMAB induce cytokinesis failure and inhibit cell proliferation in human cancer cells. Mol. Cancer Ther. 9, 995–2006. (doi:10.1158/1535-7163.MCT-10-0161) - DOI - PubMed
- Chircop M, et al. 2011. Inhibition of dynamin by dynole 34–2 induces cell death following cytokinesis failure in cancer cells. Mol. Cancer Ther. 10, 1553–1562. (doi:10.1158/1535-7163.MCT-11-0067) - DOI - PubMed
- Smith CM, Haucke V, McCluskey A, Robinson PJ, Chircop M. 2013. Inhibition of clathrin by Pitstop 2 activates the spindle assembly checkpoint and induces cell death in dividing HeLa cancer cells. Mol. Cancer 12, 4 (doi:10.1186/1476-4598-12-4) - DOI - PMC - PubMed
- Hill TA, Stewart SG, Gordon CP, Ackland SP, Gilbert J, Sauer B, Sakoff JA, McCluskey A. 2008. Norcantharidin analogues: synthesis, anticancer activity and protein phosphatase 1 and 2A inhibition. ChemMedChem. 3, 1878–1892. (doi:10.1002/cmdc.200800192) - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous