Integrative Physiology of Pneumonia - PubMed (original) (raw)
Review
Integrative Physiology of Pneumonia
Lee J Quinton et al. Physiol Rev. 2018.
Abstract
Pneumonia is a type of acute lower respiratory infection that is common and severe. The outcome of lower respiratory infection is determined by the degrees to which immunity is protective and inflammation is damaging. Intercellular and interorgan signaling networks coordinate these actions to fight infection and protect the tissue. Cells residing in the lung initiate and steer these responses, with additional immunity effectors recruited from the bloodstream. Responses of extrapulmonary tissues, including the liver, bone marrow, and others, are essential to resistance and resilience. Responses in the lung and extrapulmonary organs can also be counterproductive and drive acute and chronic comorbidities after respiratory infection. This review discusses cell-specific and organ-specific roles in the integrated physiological response to acute lung infection, and the mechanisms by which intercellular and interorgan signaling contribute to host defense and healthy respiratory physiology or to acute lung injury, chronic pulmonary disease, and adverse extrapulmonary sequelae. Pneumonia should no longer be perceived as simply an acute infection of the lung. Pneumonia susceptibility reflects ongoing and poorly understood chronic conditions, and pneumonia results in diverse and often persistent deleterious consequences for multiple physiological systems.
Figures
FIGURE 1.
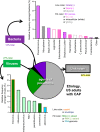
The microbial agents that cause pneumonia are numerous, diverse, and poorly understood. Data represent nonimmunocompromised adult patients hospitalized for community-acquired pneumonia (CAP). Despite intensive efforts, no microbial agent can be identified in the majority of pneumonia cases. Among the viral agents detected, no virus or type of virus was especially prominent. Among bacteria, pneumococcus was most common, but a great many species and types of bacteria were detected. [Data from Jain et al. (227).]
FIGURE 2.
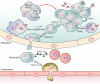
During pneumonia, macrophages have multiple critical roles in protecting the host against infection (immune resistance) and against injury (tissue resilience). The molecules identified were for illustrative purpose and do not represent an exhaustive presentation.
FIGURE 3.
During pneumonia, neutrophils have both effector (antimicrobial) and affector (immunomodulatory) roles. Effector roles include microbial elimination via phagocytosis and degranulation as well as NET formation (top right). Affector roles include activities that enhance antimicrobial activities by other cells, such as macrophage stimulation by neutrophil-derived IL-17 and TRAIL and neutrophil stimulation by IFN-γ. Other immunomodulating activities involve the recruitment of antimicrobial cells (e.g., by CXCL12, CCL17, CXCL10, CXCL2). Signals provided from apoptotic neutrophils are immunoregulatory, enhancing the resolution of inflammation. The molecules identified are for illustrative purpose and do not represent an exhaustive presentation. PhSer, phosphatidylserine.
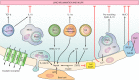
FIGURE 4.
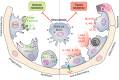
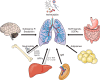
Multiple extrapulmonary organs contribute to immune resistance against pulmonary infection. The molecules identified are for illustrative purpose and do not represent an exhaustive presentation. In the few instances where signals have been identified by which pulmonary infection signals to the extrapulmonary organ, this communication is denoted in gray. APPs, acute phase proteins; SCFAs, short-chain fatty acids.
FIGURE 5.
Lungs that have experienced prior infections are different from naive lungs that have not. The T-cell population most notable in lungs from neonatal humans is regulatory T (Treg) cells, whereas the healthy lungs of adults or of laboratory animals that have experienced prior respiratory infections contain abundant resident memory T (TRM) cells. In addition, lungs that have experienced prior respiratory infections exhibit varying degrees of immunological changes including bronchus-associated lymphoid tissue (BALT), innate lymphoid cells (ILCs), and γδ-T cells. In addition, the alveolar macrophages (AM) and epithelial cells of experienced lungs behave differently from their counterparts in naive lungs, likely due to a combination of trained immunity and direction from adaptive immunity.
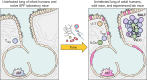
FIGURE 6.
T lymphocytes contribute differently to respiratory infection in naive and experienced hosts. A: in naive individuals, who have not previously seen relevant respiratory infections, T lymphocytes responsive to the microbe in the lungs are found in the circulating blood (red) and secondary lymphoid organs such as the spleen (purple) and lymph nodes (LN, blue). It takes several days for them to appear in the lungs and a week for their activities to be manifest. B: in contrast, in experienced hosts who have previously been infected with relevant respiratory pathogens, there are greater numbers of responsive T lymphocytes in the blood and secondary lymphoid organs, and there is a resident memory population of responsive T cells already present in the lungs before infection. These lung T cells are poised to respond more rapidly and to elaborate a broader repertoire of protective cytokines. Thus responsive T cells in experienced hosts are more numerous, localized to the right place, able to respond more quickly, and prone to becoming multifunctional, altogether leading to T cell-mediated defense in the lung that is quicker and more efficacious.
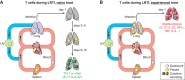
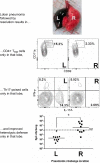
FIGURE 7.
The site of respiratory infection determines where protection resides. After pneumococcal lobar pneumonia in mice, CD4+ resident memory T (TRM) cells are found 1–2 mo later in the previously infected lobe rather than contralateral lobes. Polyclonal stimulation of cells collected from the different lobes shows that CD4+ T cells are poised to elaborate Th17 cytokines only in the previously infected lobe. Furthermore, the previously infected lobe is significantly more protected against infection by pneumococci of a different serotype compared with contralateral lobes. [Adapted from Smith et al. (459).]
FIGURE 8.
Multiple different cell types provide activities to help accomplish tissue resilience and prevent lung injury during pneumonia. Examples shown are not an exhaustive list and pictorialize some of the mechanisms described in text.
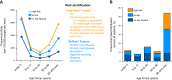
FIGURE 9.
Known risk factors for pneumonia are relevant and important, but insufficient to differentiate the susceptible from the resistant. Figure panels were generated from data reported by Pelton et al. (384), representing analyses of 3.4 million individuals in Germany from 2008 to 2012. A: pneumonia rates for individuals stratified by age group and risk factors. For all age groups, the recognized risk factors associate with elevated rates of pneumonia. Rather than susceptible versus resistant, these risk factors associate with an increased pneumonia susceptibility up to severalfold. Even those without recognized risk factors get pneumonia. For all risk groups, pneumonia rates are highest at either end of the age spectrum. B: the distribution of all pneumonia cases among age groups and risk groups. The majority of pneumonias occur in older individuals, most of whom have recognized risk factors. For younger subjects, including children and adults through age 49, more pneumonias are in subjects without recognized risk factors than in those with recognized risk.
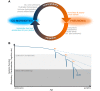
FIGURE 10.
The vicious cycle by which pneumonia drives unhealthy aging. Pneumonia susceptibility is raised by a wide variety of chronic diseases that are recognized as general risk factors for pneumonia (comorbidities). Pneumonia elicits a predisposition to and worsening of those same comorbidities. Thus comorbidities make people more likely to get pneumonia, and pneumonia precipitates and exacerbates comorbidities, which makes individuals yet more likely to get pneumonia, which yet further accelerates comorbidities, and so on. A: the self-amplifying cycle of interactions between pneumonia and comorbidities. B: the decline in physiological function driven by this self-amplifying cycle, schematically rendered to communicate the concept (not actual data).
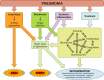
FIGURE 11.
Pneumonia sequelae. Pneumonia causes direct injury to the lungs, from a combination of microbial and inflammatory signals. When the degree of injury crosses a physiological threshold defined by blood gases, this is diagnosed as acute respiratory distress syndrome (ARDS). When infection and inflammation disseminate from the lungs and injure other organs, this is diagnosed as sepsis if severe enough to be life-threatening. ARDS and sepsis are well-recognized outcomes of pneumonia. Perhaps less well-recognized are the indirect consequences, including the predisposition to or exacerbation of ongoing chronic diseases such as COPD, atherosclerosis, cognitive decline, and more. The mechanisms driving the sequelae of pneumonia are multifactorial, including systemic inflammation and infection plus localized and diffuse aberrations involving the immune, cardiovascular, microbiome, hematologic, and nervous systems.
Similar articles
- Crosstalk Between Lung and Extrapulmonary Organs in Infection and Inflammation.
Wang Z, Pu Q, Huang C, Wu M. Wang Z, et al. Adv Exp Med Biol. 2021;1303:333-350. doi: 10.1007/978-3-030-63046-1_18. Adv Exp Med Biol. 2021. PMID: 33788201 Review. - The impact of lung microbiota dysbiosis on inflammation.
Yang D, Xing Y, Song X, Qian Y. Yang D, et al. Immunology. 2020 Feb;159(2):156-166. doi: 10.1111/imm.13139. Epub 2019 Nov 11. Immunology. 2020. PMID: 31631335 Free PMC article. Review. - Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling.
Quinton LJ, Mizgerd JP. Quinton LJ, et al. Annu Rev Physiol. 2015;77:407-30. doi: 10.1146/annurev-physiol-021014-071937. Epub 2014 Aug 13. Annu Rev Physiol. 2015. PMID: 25148693 Free PMC article. Review. - Extra-pulmonary control of respiratory defense.
Korkmaz FT, Quinton LJ. Korkmaz FT, et al. Cell Immunol. 2024 Jul-Aug;401-402:104841. doi: 10.1016/j.cellimm.2024.104841. Epub 2024 Jun 7. Cell Immunol. 2024. PMID: 38878619 Review. - Pathogenesis of severe pneumonia: advances and knowledge gaps.
Mizgerd JP. Mizgerd JP. Curr Opin Pulm Med. 2017 May;23(3):193-197. doi: 10.1097/MCP.0000000000000365. Curr Opin Pulm Med. 2017. PMID: 28221171 Free PMC article. Review.
Cited by
- Clinical risk factors and blood protein biomarkers of 10-year pneumonia risk.
Lee MM, Zuo Y, Steiling K, Mizgerd JP, Kalesan B, Walkey AJ. Lee MM, et al. PLoS One. 2024 Jul 5;19(7):e0296139. doi: 10.1371/journal.pone.0296139. eCollection 2024. PLoS One. 2024. PMID: 38968193 Free PMC article. - A methodical exploration of imaging modalities from dataset to detection through machine learning paradigms in prominent lung disease diagnosis: a review.
Kumar S, Kumar H, Kumar G, Singh SP, Bijalwan A, Diwakar M. Kumar S, et al. BMC Med Imaging. 2024 Feb 1;24(1):30. doi: 10.1186/s12880-024-01192-w. BMC Med Imaging. 2024. PMID: 38302883 Free PMC article. Review. - Sugarcoating Lung Injury: A Novel Role for High-Molecular-Weight Hyaluronan in Pneumonia.
Garantziotis S, Matalon S. Garantziotis S, et al. Am J Respir Crit Care Med. 2019 Nov 15;200(10):1197-1198. doi: 10.1164/rccm.201908-1554ED. Am J Respir Crit Care Med. 2019. PMID: 31461631 Free PMC article. No abstract available. - GL7 ligand expression defines a novel subset of CD4+ TRM cells in lungs recovered from pneumococcus.
Lyon De Ana C, Shenoy AT, Barker KA, Arafa EI, Etesami NS, Korkmaz FT, Soucy AM, Breen MP, Martin IMC, Tilton BR, Devarajan P, Crossland NA, Pihl RMF, Goltry WN, Belkina AC, Jones MR, Quinton LJ, Mizgerd JP. Lyon De Ana C, et al. Mucosal Immunol. 2023 Oct;16(5):699-710. doi: 10.1016/j.mucimm.2023.07.004. Epub 2023 Sep 14. Mucosal Immunol. 2023. PMID: 37604254 Free PMC article. - Upregulated expression of long non-coding RNA MEG3 serves as a prognostic biomarker in severe pneumonia children and its regulatory mechanism.
Guo J, Zhang N, Liu G, Zhang A, Liu X, Zheng J. Guo J, et al. Bioengineered. 2021 Dec;12(1):7120-7131. doi: 10.1080/21655979.2021.1979351. Bioengineered. 2021. PMID: 34558385 Free PMC article.
References
- Aggarwal NR, Tsushima K, Eto Y, Tripathi A, Mandke P, Mock JR, Garibaldi BT, Singer BD, Sidhaye VK, Horton MR, King LS, D’Alessio FR. Immunological priming requires regulatory T cells and IL-10-producing macrophages to accelerate resolution from severe lung inflammation. J Immunol 192: 4453–4464, 2014. doi:10.4049/jimmunol.1400146. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL136660/HL/NHLBI NIH HHS/United States
- R01 AI115053/AI/NIAID NIH HHS/United States
- R01 HL111459/HL/NHLBI NIH HHS/United States
- R61 HL137081/HL/NHLBI NIH HHS/United States
- K01 HL116768/HL/NHLBI NIH HHS/United States
- R01 HL111449/HL/NHLBI NIH HHS/United States
- R56 AI122763/AI/NIAID NIH HHS/United States
- R01 GM120060/GM/NIGMS NIH HHS/United States
- R35 HL135756/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical