Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation - PubMed (original) (raw)
Lysophospholipids induce innate immune transdifferentiation of endothelial cells, resulting in prolonged endothelial activation
Xinyuan Li et al. J Biol Chem. 2018.
Abstract
Innate immune cells express danger-associated molecular pattern (DAMP) receptors, T-cell costimulation/coinhibition receptors, and major histocompatibility complex II (MHC-II). We have recently proposed that endothelial cells can serve as innate immune cells, but the molecular mechanisms involved still await discovery. Here, we investigated whether human aortic endothelial cells (HAECs) could be transdifferentiated into innate immune cells by exposing them to hyperlipidemia-up-regulated DAMP molecules, i.e. lysophospholipids. Performing RNA-seq analysis of lysophospholipid-treated HAECs, we found that lysophosphatidylcholine (LPC) and lysophosphatidylinositol (LPI) regulate largely distinct gene programs as revealed by principal component analysis. Metabolically, LPC up-regulated genes that are involved in cholesterol biosynthesis, presumably through sterol regulatory element-binding protein 2 (SREBP2). By contrast, LPI up-regulated gene transcripts critical for the metabolism of glucose, lipids, and amino acids. Of note, we found that LPC and LPI both induce adhesion molecules, cytokines, and chemokines, which are all classic markers of endothelial cell activation, in HAECs. Moreover, LPC and LPI shared the ability to transdifferentiate HAECs into innate immune cells, including induction of potent DAMP receptors, such as CD36 molecule, T-cell costimulation/coinhibition receptors, and MHC-II proteins. The induction of these innate-immunity signatures by lysophospholipids correlated with their ability to induce up-regulation of cytosolic calcium and mitochondrial reactive oxygen species. In conclusion, lysophospholipids such as LPC and LPI induce innate immune cell transdifferentiation in HAECs. The concept of prolonged endothelial activation, discovered here, is relevant for designing new strategies for managing cardiovascular diseases.
Keywords: RNA-Seq; atherosclerosis; endothelial cell; immunometabolism; inflammation; lysophosphatidylcholine; lysophosphatidylinositol; lysophospholipid; metabolism; transdifferentiation.
© 2018 Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
Figure 1.
RNA-Seq analysis reveals that LPI induces both transient and sustained endothelial cell activation. Human aortic ECs were treated with either vehicle control (Ctr) or LPI (10 μ
m
) for 18 h, and RNA-Seq experiments were performed. n = 3 in each group. A, volcano plot showing log(-fold change (FC)) and −log10(p value) of control versus LPI treatment. Red genes indicate genes significantly changed by more than 1.4-fold by LPI. B, heat map of genes that are significantly changed by more than 1.4-fold by LPI in ECs. C, the LPC–up-regulated genes from the top regulated pathway, cellular infiltration by leukocytes, are shown. Genes that are related to innate immunity are boxed. D–H, GSEA of the gene signatures that are significantly enriched in the LPI-treated EC group. I, representative gene expression changes in different categories corresponding to the GSEA plots.
Figure 2.
LPI reprograms endothelial cell metabolism extensively besides up-regulating adhesion molecules and cytokines/chemokines in human aortic endothelial cells. Human aortic ECs were treated with either vehicle control or LPI (10 μ
m
) for 18 h, and RNA-Seq experiments were performed. The transcript level in units of transcripts per million (tpm) of the genes related to different categories, including EC adhesion molecules, cytokines/chemokines, and metabolic regulators, are shown. Box and whisker plot is shown for each sample (n = 100 bootstrap replicates from Kallisto). Lower and upper whiskers (error bars) indicate 25th and 75th percentiles, respectively.
Figure 3.
RNA-Seq analysis reveals that LPC induces both acute and sustained endothelial cell activation. Human aortic ECs were treated with either vehicle control or LPC (10 μ
m
) for 18 h, and RNA-Seq experiments were performed. n = 3 in each group. A–E, GSEA of the gene signatures that are significantly enriched in the LPC-treated EC group. F, representative gene expression changes in different categories corresponding to the GSEA plots.
Figure 4.
LPC positively regulates genes downstream of master regulator of lipid metabolism SREBP2. HAECs were challenged with LPC (10 μ
m
) for 18 h and RNA-Seq with IPA was performed. A, top regulated diseases or functions annotation of the genes that are significantly changed by LPC in HAECs. B, gene set enrichment analysis from the top LPI-regulated pathway “steroid biosynthesis” was shown. C, top upstream regulator analysis of the genes that are changed by LPC in HAECs predicted by the IPA. D, the 20 SREBP2-regulated genes that are significantly changed by LPC in HAECs. The red genes are induced by LPC and the blue genes are decreased by LPC.
Figure 5.
LPI and LPC similarly activate mitochondrial ROS, cytosolic calcium, and acute EC activation marker genes but regulate largely distinct gene programs in human aortic endothelial cells. A and B, HAECs were treated with either LPC (10 μ
m
) or LPI (10 μ
m
) for 1 h. Flow cytometry analysis with mitochondrial ROS (A) and cytosolic calcium (B) probes was performed afterward. C, GSEA of the LPI–up-regulated genes in the “calcium-mediated signaling” collection. D, principal component analysis showing the global transcription profile relationship among control, LPI, and LPC. E, heat map showing the similarities and differences between LPC-regulated and LPI-regulated genes. F, Venn diagram showing the number of LPC and LPI co-up-regulated genes. G, top enriched pathways of the LPI and LPC co-up-regulated genes (58 genes from F) as determined by Ingenuity Pathway Analysis. H, the LPC and LPI co-up-regulated genes from their top regulated pathway, attraction of leukocytes (in G), are shown. I, comparison of LPS- and lysophospholipid-induced endothelial activation. Red numbers indicate gene expression -fold changes that are greater than 1.4-fold. For all panels, data are expressed as mean ± S.D. (error bars). **, p < 0.01; ***, p < 0.001.
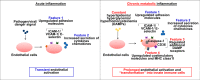
Figure 6.
A new working model. Left, during acute inflammation, a danger signal from pathogen or virus infection induces transient endothelial cell activation as characterized by two features, up-regulated adhesion molecule expression and increased secretion of cytokines and chemokines. Right, in the process of chronic metabolic inflammation during cardiovascular disease development, constant stimulation from endogenous metabolic DAMPs, such as lysophospholipids (during hyperlipidemia), glucose (during hyperglycemia), and homocysteine (during hyperhomocysteinemia), transform endothelial cells into innate immune cells and induce prolonged endothelial cell activation. The innate reprogramming of endothelial cells is characterized not only by up-regulation of cytokine/chemokines and adhesion molecule gene expression but also by up-regulation of additional DAMP receptors, such as caspase-1 and CD36, as well as up-regulation of costimulatory molecules and MHC class II molecules.
Similar articles
- Anti-inflammatory cytokines IL-35 and IL-10 block atherogenic lysophosphatidylcholine-induced, mitochondrial ROS-mediated innate immune activation, but spare innate immune memory signature in endothelial cells.
Li X, Fang P, Sun Y, Shao Y, Yang WY, Jiang X, Wang H, Yang X. Li X, et al. Redox Biol. 2020 Jan;28:101373. doi: 10.1016/j.redox.2019.101373. Epub 2019 Nov 6. Redox Biol. 2020. PMID: 31731100 Free PMC article. - Mitochondrial Reactive Oxygen Species Mediate Lysophosphatidylcholine-Induced Endothelial Cell Activation.
Li X, Fang P, Li Y, Kuo YM, Andrews AJ, Nanayakkara G, Johnson C, Fu H, Shan H, Du F, Hoffman NE, Yu D, Eguchi S, Madesh M, Koch WJ, Sun J, Jiang X, Wang H, Yang X. Li X, et al. Arterioscler Thromb Vasc Biol. 2016 Jun;36(6):1090-100. doi: 10.1161/ATVBAHA.115.306964. Epub 2016 Apr 28. Arterioscler Thromb Vasc Biol. 2016. PMID: 27127201 Free PMC article. - Lysophosphatidylcholine promotes SREBP-2 activation via rapid cholesterol efflux and SREBP-2-independent cytokine release in human endothelial cells.
Morita M, Sekine A, Urano Y, Nishimura T, Takabe W, Arai H, Hamakubo T, Kodama T, Noguchi N. Morita M, et al. J Biochem. 2015 Oct;158(4):331-8. doi: 10.1093/jb/mvv044. Epub 2015 May 21. J Biochem. 2015. PMID: 25998247 - Endothelial Damage, Inflammation and Immunity in Chronic Kidney Disease.
Diaz-Ricart M, Torramade-Moix S, Pascual G, Palomo M, Moreno-Castaño AB, Martinez-Sanchez J, Vera M, Cases A, Escolar G. Diaz-Ricart M, et al. Toxins (Basel). 2020 Jun 1;12(6):361. doi: 10.3390/toxins12060361. Toxins (Basel). 2020. PMID: 32492843 Free PMC article. Review. - Phospholipid-derived lysophospholipids in (patho)physiology.
Prabutzki P, Schiller J, Engel KM. Prabutzki P, et al. Atherosclerosis. 2024 Nov;398:118569. doi: 10.1016/j.atherosclerosis.2024.118569. Epub 2024 Aug 23. Atherosclerosis. 2024. PMID: 39227208 Review.
Cited by
- Canonical Secretomes, Innate Immune Caspase-1-, 4/11-Gasdermin D Non-Canonical Secretomes and Exosomes May Contribute to Maintain Treg-Ness for Treg Immunosuppression, Tissue Repair and Modulate Anti-Tumor Immunity via ROS Pathways.
Ni D, Tang T, Lu Y, Xu K, Shao Y, Saaoud F, Saredy J, Liu L, Drummer C 4th, Sun Y, Hu W, Lopez-Pastrana J, Luo JJ, Jiang X, Choi ET, Wang H, Yang X. Ni D, et al. Front Immunol. 2021 May 18;12:678201. doi: 10.3389/fimmu.2021.678201. eCollection 2021. Front Immunol. 2021. PMID: 34084175 Free PMC article. - Innate immunity of vascular smooth muscle cells contributes to two-wave inflammation in atherosclerosis, twin-peak inflammation in aortic aneurysms and trans-differentiation potential into 25 cell types.
Yang Q, Saaoud F, Lu Y, Pu Y, Xu K, Shao Y, Jiang X, Wu S, Yang L, Tian Y, Liu X, Gillespie A, Luo JJ, Shi XM, Zhao H, Martinez L, Vazquez-Padron R, Wang H, Yang X. Yang Q, et al. Front Immunol. 2024 Jan 24;14:1348238. doi: 10.3389/fimmu.2023.1348238. eCollection 2023. Front Immunol. 2024. PMID: 38327764 Free PMC article. - Increasing Upstream Chromatin Long-Range Interactions May Favor Induction of Circular RNAs in LysoPC-Activated Human Aortic Endothelial Cells.
Li A, Sun Y, Drummer C 4th, Lu Y, Yu D, Zhou Y, Li X, Pearson SJ, Johnson C, Yu C, Yang WY, Mastascusa K, Jiang X, Sun J, Rogers T, Hu W, Wang H, Yang X. Li A, et al. Front Physiol. 2019 Apr 18;10:433. doi: 10.3389/fphys.2019.00433. eCollection 2019. Front Physiol. 2019. PMID: 31057422 Free PMC article. - End-stage renal disease is different from chronic kidney disease in upregulating ROS-modulated proinflammatory secretome in PBMCs - A novel multiple-hit model for disease progression.
Zhang R, Saredy J, Shao Y, Yao T, Liu L, Saaoud F, Yang WY, Sun Y, Johnson C, Drummer C 4th, Fu H, Lu Y, Xu K, Liu M, Wang J, Cutler E, Yu D, Jiang X, Li Y, Li R, Wang L, Choi ET, Wang H, Yang X. Zhang R, et al. Redox Biol. 2020 Jul;34:101460. doi: 10.1016/j.redox.2020.101460. Epub 2020 Feb 20. Redox Biol. 2020. PMID: 32179051 Free PMC article. - Hyperlipidemia May Synergize with Hypomethylation in Establishing Trained Immunity and Promoting Inflammation in NASH and NAFLD.
Drummer CIV, Saaoud F, Sun Y, Atar D, Xu K, Lu Y, Shao Y, Johnson C, Liu L, Shen H, Jhala NC, Jiang X, Wang H, Yang X. Drummer CIV, et al. J Immunol Res. 2021 Nov 23;2021:3928323. doi: 10.1155/2021/3928323. eCollection 2021. J Immunol Res. 2021. PMID: 34859106 Free PMC article.
References
- Yin Y., Li X., Sha X., Xi H., Li Y. F., Shao Y., Mai J., Virtue A., Lopez-Pastrana J., Meng S., Tilley D. G., Monroy M. A., Choi E. T., Thomas C. J., Jiang X., et al. (2015) Early hyperlipidemia promotes endothelial activation via a caspase-1-sirtuin 1 pathway. Arterioscler. Thromb. Vasc. Biol. 35, 804–816 10.1161/ATVBAHA.115.305282 - DOI - PMC - PubMed
- Li X., Fang P., Li Y., Kuo Y. M., Andrews A. J., Nanayakkara G., Johnson C., Fu H., Shan H., Du F., Hoffman N. E., Yu D., Eguchi S., Madesh M., Koch W. J., et al. (2016) Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler. Thromb. Vasc. Biol. 36, 1090–1100 10.1161/ATVBAHA.115.306964 - DOI - PMC - PubMed
- Li X., Fang P., Yang W. Y., Chan K., Lavallee M., Xu K., Gao T., Wang H., and Yang X. (2017) Mitochondrial ROS, uncoupled from ATP synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells. Can. J. Physiol. Pharmacol. 95, 247–252 10.1139/cjpp-2016-0515 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL138749/HL/NHLBI NIH HHS/United States
- R01 DK113775/DK/NIDDK NIH HHS/United States
- R01 HL117654/HL/NHLBI NIH HHS/United States
- R01 HL130233/HL/NHLBI NIH HHS/United States
- R01 HL131460/HL/NHLBI NIH HHS/United States
- R01 HL110764/HL/NHLBI NIH HHS/United States
- R01 DK104116/DK/NIDDK NIH HHS/United States
- R01 HL132399/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials