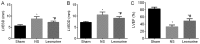Leonurine protects cardiac function following acute myocardial infarction through anti‑apoptosis by the PI3K/AKT/GSK3β signaling pathway - PubMed (original) (raw)
Leonurine protects cardiac function following acute myocardial infarction through anti‑apoptosis by the PI3K/AKT/GSK3β signaling pathway
Lin Xu et al. Mol Med Rep. 2018 Aug.
Erratum in
- [Corrigendum] Leonurine protects cardiac function following acute myocardial infarction through anti‑apoptosis by the PI3K/AKT/GSK3β signaling pathway.
Xu L, Jiang X, Wei F, Zhu H. Xu L, et al. Mol Med Rep. 2023 Mar;27(3):65. doi: 10.3892/mmr.2023.12952. Epub 2023 Feb 3. Mol Med Rep. 2023. PMID: 36734264 Free PMC article.
Abstract
Leonurine is a compound derived from Herba leonuri, which has been reported to protect cardiac tissue against ischemic injury via antioxidant and anti‑apoptosis effects. The present study investigated whether these effects may be applied to acute myocardial infarction (MI) and examined the underlying mechanisms of leonurine treatment. A rat model of MI was induced by coronary artery ligation. Leonurine was administered at 15 mg/kg/day by oral gavage following the onset of MI. Rats in the sham group and the saline group were administered with an equal volume of saline. Echocardiography, Masson's trichrome staining, and terminal‑deoxynucleotidyl transferase‑mediated dUTP nick end labeling assays were performed 28 days post MI. The expression of B‑cell lymphoma‑2 and Bax were assessed by western blot analysis and reverse transcription‑quantitative polymerase chain reaction. Phosphoinositide 3‑kinase (PI3K), protein kinase B and glycogen synthase kinase‑3β (GSK3β) protein expression were investigated by western blot analysis. Leonurine significantly alleviated collagen deposition and MI size, inhibited cell apoptosis and improved myocardial function. This was accompanied by significantly increased levels of phosphorylated (p)‑PI3K, p‑AKT, p‑GSK3β and Bcl‑2, as well as significantly decreased levels of caspase3, cleaved‑caspase3 and Bax following MI. The results demonstrated that leonurine exerts potent cardio‑protective effects in a rat model of MI by inducing anti‑apoptotic effects by activating the PI3K/AKT/GSK3β signaling pathway.
Figures
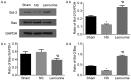
Figure 1.
Echocardiographic evaluation of left ventricular function, including (A) LVEDD, (B) LVESD and (C) LVEF 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=12 in each group. LVESD, left ventricular end-systolic diameter; LVEDD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fractional; NS, saline group.
Figure 2.
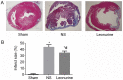
Infarct size 28 days after myocardial infarction. (A) Representative pictures of left ventricles from each group after Masson's Trichrome staining (magnification, ×10). (B) Infarct size as percentages at 28 days. Infarct size is calculated from the ratio of surface area of infarct wall and the entire surface area of the left ventricle. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. NS, saline group.
Figure 3.
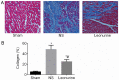
Collagen content 28 days after myocardial infarction. (A) Representative pictures of left ventricles from each group after Masson's Trichrome staining (magnification, ×200). (B) Collagen content as percentages at 28 days. Collagen content is calculated from the ratio of the collagen area to the area of the entire high-power field. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. NS, saline group.
Figure 4.
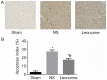
Cardiomyocyte apoptosis 28 days after myocardial infarction. (A) Immunohistochemistry of TUNEL staining in the border zone (Magnifiaction, ×200). (B) The apoptotic index was quantified at 28 days. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS; n=6 in each group. NS, saline group.
Figure 5.
The level of Bcl-2 and Bax mRNA 28 days after MI. (A) The level of Bcl-2 and (B) Bax mRNA 28 days after MI. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS; n=6 in each group. NS, saline group; Bcl-2, B-cell lymphoma 2; MI, myocardial infarction.
Figure 6.
Representative immunoblots and quantified results of (A) Bcl-2 and Bax, (B) p-PI3K and PI3K, (C) p-AKT and AKT proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. Representative immunoblots and quantified results of (D) p-GSK3β and GSK3β and (E) Caspase3 and Cleaved-caspase3 proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. PI3K, phosphoinositide 3-kinase; GSK3β, glycogen synthase kinase-3β.
Figure 6.
Representative immunoblots and quantified results of (A) Bcl-2 and Bax, (B) p-PI3K and PI3K, (C) p-AKT and AKT proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. Representative immunoblots and quantified results of (D) p-GSK3β and GSK3β and (E) Caspase3 and Cleaved-caspase3 proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. PI3K, phosphoinositide 3-kinase; GSK3β, glycogen synthase kinase-3β.
Figure 6.
Representative immunoblots and quantified results of (A) Bcl-2 and Bax, (B) p-PI3K and PI3K, (C) p-AKT and AKT proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. Representative immunoblots and quantified results of (D) p-GSK3β and GSK3β and (E) Caspase3 and Cleaved-caspase3 proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. PI3K, phosphoinositide 3-kinase; GSK3β, glycogen synthase kinase-3β.
Figure 6.
Representative immunoblots and quantified results of (A) Bcl-2 and Bax, (B) p-PI3K and PI3K, (C) p-AKT and AKT proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. Representative immunoblots and quantified results of (D) p-GSK3β and GSK3β and (E) Caspase3 and Cleaved-caspase3 proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. PI3K, phosphoinositide 3-kinase; GSK3β, glycogen synthase kinase-3β.
Figure 6.
Representative immunoblots and quantified results of (A) Bcl-2 and Bax, (B) p-PI3K and PI3K, (C) p-AKT and AKT proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. Representative immunoblots and quantified results of (D) p-GSK3β and GSK3β and (E) Caspase3 and Cleaved-caspase3 proteins by western blot analysis 28 days after myocardial infarction. The data are expressed as the mean ± standard deviation. *P<0.05 vs. sham, #P<0.05 vs. NS. n=6 in each group. PI3K, phosphoinositide 3-kinase; GSK3β, glycogen synthase kinase-3β.
Similar articles
- Effects of dexmedetomidine postconditioning on myocardial ischemia and the role of the PI3K/Akt-dependent signaling pathway in reperfusion injury.
Cheng XY, Gu XY, Gao Q, Zong QF, Li XH, Zhang Y. Cheng XY, et al. Mol Med Rep. 2016 Jul;14(1):797-803. doi: 10.3892/mmr.2016.5345. Epub 2016 May 24. Mol Med Rep. 2016. PMID: 27221008 Free PMC article. - Syringic acid mitigates myocardial ischemia reperfusion injury by activating the PI3K/Akt/GSK-3β signaling pathway.
Liu G, Zhang BF, Hu Q, Liu XP, Chen J. Liu G, et al. Biochem Biophys Res Commun. 2020 Oct 15;531(2):242-249. doi: 10.1016/j.bbrc.2020.07.047. Epub 2020 Aug 12. Biochem Biophys Res Commun. 2020. PMID: 32798018 - Apoptosis of cardiomyocytes in diabetic cardiomyopathy involves overexpression of glycogen synthase kinase-3β.
Wu W, Liu X, Han L. Wu W, et al. Biosci Rep. 2019 Jan 3;39(1):BSR20171307. doi: 10.1042/BSR20171307. Print 2019 Jan 31. Biosci Rep. 2019. PMID: 30237226 Free PMC article. - Pioneering therapies for post-infarction angiogenesis: Insight into molecular mechanisms and preclinical studies.
Chen C, Wang J, Liu C, Hu J, Liu L. Chen C, et al. Biomed Pharmacother. 2023 Oct;166:115306. doi: 10.1016/j.biopha.2023.115306. Epub 2023 Aug 10. Biomed Pharmacother. 2023. PMID: 37572633 Review. - Immunomodulatory Action of Substituted 1,3,4-Thiadiazines on the Course of Myocardial Infarction.
Sarapultsev AP, Vassiliev PM, Sarapultsev PA, Chupakhin ON, Ianalieva LR, Sidorova LP. Sarapultsev AP, et al. Molecules. 2018 Jul 2;23(7):1611. doi: 10.3390/molecules23071611. Molecules. 2018. PMID: 30004445 Free PMC article. Review.
Cited by
- Integrative Analysis of Expression Profiles of MicroRNAs and mRNAs in Treatment of Acute Myocardial Infarction with Compound Longmaining Decoction.
Bu D, Su Z, Meng M, Wang C. Bu D, et al. Med Sci Monit. 2019 Nov 28;25:9028-9041. doi: 10.12659/MSM.917925. Med Sci Monit. 2019. PMID: 31776323 Free PMC article. - Decoy receptor-3 regulates inflammation and apoptosis via PI3K/AKT signaling pathway in coronary heart disease.
Chen X, Wang R, Chen W, Lai L, Li Z. Chen X, et al. Exp Ther Med. 2019 Apr;17(4):2614-2622. doi: 10.3892/etm.2019.7222. Epub 2019 Jan 30. Exp Ther Med. 2019. PMID: 30906453 Free PMC article. - Supplementation of Saponins from Leaves of Panax quinquefolius Mitigates Cisplatin-Evoked Cardiotoxicity via Inhibiting Oxidative Stress-Associated Inflammation and Apoptosis in Mice.
Xing JJ, Hou JG, Liu Y, Zhang RB, Jiang S, Ren S, Wang YP, Shen Q, Li W, Li XD, Wang Z. Xing JJ, et al. Antioxidants (Basel). 2019 Sep 1;8(9):347. doi: 10.3390/antiox8090347. Antioxidants (Basel). 2019. PMID: 31480577 Free PMC article. - CLEC3B protects H9c2 cardiomyocytes from apoptosis caused by hypoxia via the PI3K/Akt pathway.
Lv F, Wang Z, Huang Y, Si A, Chen Y. Lv F, et al. Braz J Med Biol Res. 2020;53(9):e9693. doi: 10.1590/1414-431x20209693. Epub 2020 Jul 17. Braz J Med Biol Res. 2020. PMID: 32696821 Free PMC article. - The co-treatment of rosuvastatin with dapagliflozin synergistically inhibited apoptosis via activating the PI3K/AKt/mTOR signaling pathway in myocardial ischemia/reperfusion injury rats.
Gong L, Wang X, Pan J, Zhang M, Liu D, Liu M, Li L, An F. Gong L, et al. Open Med (Wars). 2020 Dec 11;15(1):47-57. doi: 10.1515/med-2021-0005. eCollection 2021. Open Med (Wars). 2020. PMID: 33385063 Free PMC article.
References
- Zhu YZ, Chong CL, Chuah SC, Huang SH, Nai HS, Tong HT, Whiteman M, Moore PK. Cardioprotective effects of nitroparacetamol and paracetamol in acute phase of myocardial infarction in experimental rats. Am J Physiol Heart Circ Physiol. 2006;290:H517–H524. doi: 10.1152/ajpheart.00572.2005. - DOI - PubMed
- Liu C, Guo W, Maerz S, Gu X, Zhu Y. 3,5-Dimethoxy-4-(3-(2-carbonyl-ethyldisulfanyl)-propionyl)-benzoic acid 4-guanidino-butyl ester: A novel twin drug that prevents primary cardiac myocytes from hypoxia-induced apoptosis. Eur J Pharmacol. 2013;700:118–126. doi: 10.1016/j.ejphar.2012.11.028. - DOI - PubMed
- Gallogly MM, Shelton MD, Qanungo S, Pai HV, Starke DW, Hoppel CL, Lesnefsky EJ, Mieyal JJ. Glutaredoxin regulates apoptosis in cardiomyocytes via NFkappaB targets Bcl-2 and Bcl-xL: Implications for cardiac aging. Antioxid Redox Signal. 2010;12:1339–1353. doi: 10.1089/ars.2009.2791. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials