Transcorneal Electrical Stimulation Inhibits Retinal Microglial Activation and Enhances Retinal Ganglion Cell Survival After Acute Ocular Hypertensive Injury - PubMed (original) (raw)
Transcorneal Electrical Stimulation Inhibits Retinal Microglial Activation and Enhances Retinal Ganglion Cell Survival After Acute Ocular Hypertensive Injury
Lin Fu et al. Transl Vis Sci Technol. 2018.
Abstract
Purpose: To investigate the effect of transcorneal electrical stimulation (TcES) on retinal ganglion cell (RGC) function and survival after acute ocular hypertension-related retinal injury in gerbil eyes.
Methods: Gerbil eyes were subjected to acute ocular hypertensive injury (80 mm Hg for 60 minutes). In the treatment group, TcES was applied to the surgical eye immediately and then twice weekly for a total of 1 month. In the control group, sham TcES was given to the surgical eye at the same time points. Retinal function was assessed and compared between groups using flash electroretinography. For histological analysis, the number of RGC and microglial cells were counted by immunofluorescence staining after the gerbils were sacrificed on day 7 and day 28. Real-time polymerase chain reaction and western blot analysis were conducted to compare expression of interleukin (IL)-10, IL-6, COX-2, tumor necrosis factor (TNF)-α, and NF-κB phosphorylation among groups.
Results: TcES-treated eyes had significantly higher RGC survival at 1 month compared to controls. This was associated with RGC function. Furthermore, TcES-treated eyes were shown to have increased IL-10 expression, with a corresponding reduction in IL-6 and COX-2 expression as well as reduction in NF-κB phosphorylation. This was associated with a suppression in microglial cell activation in TcES-treated eyes.
Conclusions: Early treatment with TcES in gerbils protected the RGC from secondary damage and preserved retinal function in acute ocular hypertensive injury through modulation of the microglial-cell activated local inflammatory response.
Translational relevance: Our study strengthens the argument for translating TcES as a viable treatment in acute glaucoma.
Keywords: acute ocular hypertensive injury; anti-inflammatory; neuroprotection; transcorneal electrical stimulation.
Figures
Figure 1
Setting of AOH induction. (a) Anterior chamber infusion of BSS through a needle to the gerbil's eye. (b) Intraocular view of the infusion under microscope. (c) The BSS was hanged at a height of 2.6 m to the gerbil's eye.
Figure 2
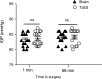
IOP readings during AOH. There were no significant differences (P > 0.05) of the IOP readings between the TcES group and sham group at the beginning or the end of the surgery. ns, no significance.
Figure 3
Retinal function reduced after AOH but was improved by TcES. (a), (e) Representative ERG responses at 1 week and 1 month. (b), (c), (d), (f), (g), (h) Averaged amplitudes of 1 week and 1 month a-wave, b-wave, and PhNR. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05. n, number of retinas in each group; ns, no significance.
Figure 4
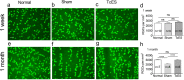
Protection of RGCs by TcES. (a–c) Brn-3a stained RGCs in retinal flat mount of normal, sham, and TcES group at 1 week. (e–g) Brn-3a stained RGCs in retinal flat mount of normal, sham, and TcES group at 1 month. (d, h) Mean density of the RGCs in the retina flat mount of 1 week and 1 month results. Scale bar: 40 μm. ****P < 0.0001. n, number of retinas in each group; ns, no significance.
Figure 5
TcES suppressed the inflammatory factors. (a, b) Western blot results of NF-κB phosphorylation level. (c–e) Western blot results of IL-6, COX-2, and IL-10 protein level. ***P < 0.001, **P < 0.01, *P < 0.05. n, number of retinas in each group; ns, no significance.
Figure 6
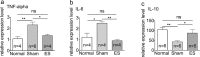
Downregulation of mRNA expression level in inflammatory genes by TcES. (a–c) mRNA expression in TNF-α, IL-6, and IL-10 genes. **P < 0.01, *P < 0.05. n, number of retinas in each group; ns, no significance.
Figure 7
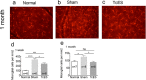
TcES inhibited the activation of microglial cells in RGC layer. (a–c) Fluorescent images of Iba-1 stained microglial cells in retinal whole mounts. (d, e) Quantification of microglia density at 1 week and 1 month after AOH. Scale bar: 50 μm. ****P < 0.0001; ***P < 0.001, *P < 0.05. n, number of retinas in each group; ns, no significance.
Similar articles
- Neuroprotective effect of transcorneal electrical stimulation on light-induced photoreceptor degeneration.
Ni YQ, Gan DK, Xu HD, Xu GZ, Da CD. Ni YQ, et al. Exp Neurol. 2009 Oct;219(2):439-52. doi: 10.1016/j.expneurol.2009.06.016. Epub 2009 Jul 2. Exp Neurol. 2009. PMID: 19576889 - Transcorneal electrical stimulation promotes survival of retinal ganglion cells after optic nerve transection in rats accompanied by reduced microglial activation and TNF-α expression.
Yin H, Yin H, Zhang W, Miao Q, Qin Z, Guo S, Fu Q, Ma J, Wu F, Yin J, Yang Y, Fang X. Yin H, et al. Brain Res. 2016 Nov 1;1650:10-20. doi: 10.1016/j.brainres.2016.08.034. Epub 2016 Aug 25. Brain Res. 2016. PMID: 27569587 - Inhibition of the cysteinyl leukotriene pathways increases survival of RGCs and reduces microglial activation in ocular hypertension.
Trost A, Motloch K, Koller A, Bruckner D, Runge C, Schroedl F, Bogner B, Kaser-Eichberger A, Strohmaier C, Ladek AM, Preishuber-Pfluegl J, Brunner SM, Aigner L, Reitsamer HA. Trost A, et al. Exp Eye Res. 2021 Dec;213:108806. doi: 10.1016/j.exer.2021.108806. Epub 2021 Oct 27. Exp Eye Res. 2021. PMID: 34715090 - [Nonarteritic ischemic optic neuropathy animal model and its treatment applications].
Chuman H. Chuman H. Nippon Ganka Gakkai Zasshi. 2014 Apr;118(4):331-61. Nippon Ganka Gakkai Zasshi. 2014. PMID: 24864434 Review. Japanese. - [A challenge to primary open-angle glaucoma including normal-pressure. Clinical problems and their scientific solution].
Sugiyama K. Sugiyama K. Nippon Ganka Gakkai Zasshi. 2012 Mar;116(3):233-67; discussion 268. Nippon Ganka Gakkai Zasshi. 2012. PMID: 22568103 Review. Japanese.
Cited by
- Electrical Stimulation Induces Retinal Müller Cell Proliferation and Their Progenitor Cell Potential.
Enayati S, Chang K, Achour H, Cho KS, Xu F, Guo S, Z Enayati K, Xie J, Zhao E, Turunen T, Sehic A, Lu L, Utheim TP, Chen DF. Enayati S, et al. Cells. 2020 Mar 23;9(3):781. doi: 10.3390/cells9030781. Cells. 2020. PMID: 32210151 Free PMC article. - Therapeutic Strategies for Attenuation of Retinal Ganglion Cell Injury in Optic Neuropathies: Concepts in Translational Research and Therapeutic Implications.
Fu L, Kwok SS, Chan YK, Ming Lai JS, Pan W, Nie L, Shih KC. Fu L, et al. Biomed Res Int. 2019 Nov 11;2019:8397521. doi: 10.1155/2019/8397521. eCollection 2019. Biomed Res Int. 2019. PMID: 31828134 Free PMC article. Review. - Reversibility of visual field defects through induction of brain plasticity: vision restoration, recovery and rehabilitation using alternating current stimulation.
Sabel BA, Gao Y, Antal A. Sabel BA, et al. Neural Regen Res. 2020 Oct;15(10):1799-1806. doi: 10.4103/1673-5374.280302. Neural Regen Res. 2020. PMID: 32246620 Free PMC article. Review. - Efficacy and safety of transdermal electrical stimulation in patients with nonarteritic anterior ischemic optic neuropathy.
Miura G, Fujiwara T, Ozawa Y, Shiko Y, Kawasaki Y, Nizawa T, Tatsumi T, Kurimoto T, Mori S, Nakamura M, Hanaoka H, Baba T, Yamamoto S. Miura G, et al. Bioelectron Med. 2023 Oct 25;9(1):22. doi: 10.1186/s42234-023-00125-2. Bioelectron Med. 2023. PMID: 37876021 Free PMC article. - Mechanisms of electrical stimulation in eye diseases: A narrative review.
Liu J, Ma AKH, So KF, Lee VWH, Chiu K. Liu J, et al. Adv Ophthalmol Pract Res. 2022 May 5;2(2):100060. doi: 10.1016/j.aopr.2022.100060. eCollection 2022 Aug-Sep. Adv Ophthalmol Pract Res. 2022. PMID: 37846384 Free PMC article. Review.
References
- Quek DT, Koh VT, Tan GS, Perera SA, Wong TT, Aung T. . Blindness and long-term progression of visual field defects in chinese patients with primary angle-closure glaucoma. . 2011; 152: 463– 469. - PubMed
- Tan AM, Loon SC, Chew PT. . Outcomes following acute primary angle closure in an Asian population. . 2009; 37: 467– 472. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials