A critical revision of the fossil record, stratigraphy and diversity of the Neogene seal genus Monotherium (Carnivora, Phocidae) - PubMed (original) (raw)
A critical revision of the fossil record, stratigraphy and diversity of the Neogene seal genus Monotherium (Carnivora, Phocidae)
Leonard Dewaele et al. R Soc Open Sci. 2018.
Abstract
Historically, Monotherium had been one of the few genera of extinct Phocidae (true seals) that served as a wastebin taxon. Consequently, it did neither aid in understanding phylogenetic relationships of extinct Phocidae, nor in understanding seal diversity in deep time. This urged the reassessment of the genus. Before our review, Monotherium included five different species: Monotherium aberratum, Monotherium affine, and Monotherium delognii from Belgium; Monotherium gaudini from Italy; and Monotherium? wymani from the east coast USA. In this work we redescribe the fossil record of the genus, retaining the type species M. delognii. Monotherium aberratum and M. affine are reassigned to the new phocine genus Frisiphoca. Monotherium gaudini is renamed and considered a stem-monachine (Noriphoca gaudini). The holotype of the monachine M.? wymani requires further study pending the discovery of new fossil material that could be attributed to the same taxon. Reinvestigating the stratigraphic context reveals that N. gaudini most likely represents one of the two oldest named phocid seals, or even the oldest, dated to the late Oligocene-earliest Miocene. Our results allow questioning the widespread idea that Phocidae originated in the western Atlantic and better appreciate their palaeobiogeography during the late Oligocene-Miocene interval in the North Atlantic realm.
Keywords: Monotherium; Neogene; North Atlantic; North Sea Basin; Phocidae.
Conflict of interest statement
The authors declare that there are no competing interests.
Figures
Figure 1.
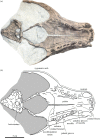
Holotype skull of the stem monachine Noriphoca gaudini, MSNUN123, presumably from the late Oligocene–early Miocene Lepidocyclina Limestone of the Bolognano Formation near Roccamorice, Italy, and originally described as Phoca gaudini by Guiscardi ([7]: plate 1). Original drawing from Guiscardi [7] (a), and line drawing (b). Skull in ventral view. Sediment and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Figure 2.
Holotype skull of the stem monachine Noriphoca gaudini, MSNUN123, presumably from the late Oligocene–early Miocene Lepidocyclina Limestone of the Bolognano Formation near Roccamorice, Italy, and originally described as Phoca gaudini by Guiscardi ([7]: plate 2), also including isolated teeth originally assigned to P. gaudini. Original drawing from Guiscardi [7] (a_–_e), and line drawing (f_–_j). Skull in right lateral view (a,f), and snout in anterior view (b,g). Corresponding scale bar equals 5 cm. Isolated right postcanine tooth in lingual (c,h), labial (d,i) and occlusal (e,j) view. Corresponding scale bar equals 2 cm. Sediment and obliterated parts are indicated in grey.
Figure 3.
Line drawings of the holotype tympanic bulla MCZ 8741 of Monotherium? wymani (?Calvert Formation at Richmond, Virginia) in ventral view. After figures from Ray [15]. Broken and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Figure 4.
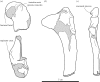
Lectotype right humerus IRSNB 1191-M266 of the stem phocine Frisiphoca aberratum from the ‘Diestian’ of the ‘third section’ at Borgerhout, Antwerp, in posterior (a), medial (b), anterior (c) and lateral (d) view. Lectotype right humerus IRSNB 1118-M260 of the stem phocine Frisiphoca affine from the ‘Diestian’ of the ‘third section’ at Deurne, Antwerp, in posterior (e), medial (f), anterior (g) and lateral (h) view. Corresponding labelled drawings of right humerus IRSNB 1191-M266 of Frisiphoca aberratum in posterior (i), medial (j), anterior (k) and lateral (l) view; and lectotype right humerus IRSNB 1118-M260 of Frisiphoca affine in posterior (m), medial (n), anterior (o) and lateral (p) view. Broken and obliterated parts are indicated in grey. Scale bar equals 10 cm.
Figure 5.
Line drawings of right humerus USNM 214625 (a) (Phocinae cf. Frisiphoca aberratum) (St Marys Formation of the Gay Head Greensands at Martha's Vineyard, Massachusetts) in lateral (a) view; and left ulna USNM 187410 (b,c) (Phocinae cf. Frisiphoca affine) (?Calvert Formation at Richmond, Virginia) in medial (b) and anterior (c) view. After figures from Ray [15]. USNM 214625 was considered ?Monatherium aberratum, and USNM 187410 was considered Monotherium? wymani. Broken and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Figure 6.
Right ulna IRSNB 1121-M261a assigned here to Phocidae cf. Frisiphoca affine (originally Monatherium affine by Van Beneden [17]) from the ‘Diestian’ of the ‘third section’ at Borgerhout, Antwerp, in lateral (a), anterior (b) and medial (c) view. Scale bar equals 5 cm.
Figure 7.
Left astragalus IRSNB 1126-M262 assigned here to Phocidae cf. Frisiphoca affine (originally Monatherium affine by Van Beneden [17]) from the ‘Diestian’ of the ‘third section’ at Borgerhout, Antwerp, in lateral (a), medial (b) and dorsal (c) view. Corresponding labelled drawings of IRSNB 1126-M262 in lateral (d), medial (e) and dorsal (f) view. Broken and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Figure 8.
Right calcaneum IRSNB 1125-M263 assigned here to Phocidae cf. Frisiphoca affine (originally Monatherium affine by Van Beneden [17]) from the ‘Diestian’ of the ‘third section’ at Borgerhout, Antwerp, in lateral (a), medial (b) and dorsal (c) view. Corresponding labelled drawings of IRSNB 1125-M263 in lateral (d), medial (e) and dorsal (f) view. Broken and obliterated parts are indicated in grey. Scale bar equals 5 cm.
Figure 9.
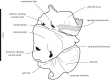
Partial pelvis IRSNB 1153-M257a, b including a sacrum (a) and a left innominate (b), assigned to Monatherium delognii (Van Beneden [17]) from the ‘Diestian’ of the ‘third section’ at Deurne, Antwerp in anterior (a), left lateral (b) and dorsal (c) view. Scale bar equals 10 cm.
Figure 10.
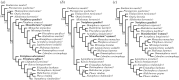
Phylogenetic tree resulting from the (a) first, (b) second and (c) third analysis. (a) The first analysis includes Frisiphoca aberratum, Frisiphoca affine, Monotherium? wymani and Noriphoca gaudini. 50% majority consensus tree of 29 most parsimonious trees without equal weighting of homoplastic characters. (b) The second analysis includes M.? wymani and N. gaudini. 50% majority consensus tree of 75 most parsimonious trees without equal weighting of homoplastic characters. (c) The third analysis includes M.? wymani and N. gaudini (a). 50% majority consensus tree of 174 most parsimonious trees with equal weighting of homoplastic characters. Frisiphoca aberratum, F. affine, M.? wymani and N. gaudini highlighted in bold. Extinct taxa are indicated by a dagger. All bootstrap values equal to or higher than 50 (after 10 000 replicates) are shown.
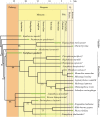
Figure 11.
Phylogenetic tree resulting from the fourth analysis, excluding Frisiphoca aberratum, Frisiphoca affine and Monotherium? wymani. 50% majority consensus tree of the six most parsimonious trees. The age ranges for extinct OTUs are expressed as a green bar over each relevant terminal branch. Bootstrap values exceeding 50% are indicated on the relevant branches. Geochronological ages for the included species, whenever fossil or subfossil specimens have been documented: Acrophoca longirostris [42], Allodesmus kernensis [76] Enaliarctos mealsi [52], Devinophoca claytoni [30], Erignathus barbatus [77,78], Hadrokirus martini [8], Halichoerus grypus [79], Homiphoca capensis [80], Hydrurga leptonyx [18,81], Kawas benegasorum [82], Leptophoca proxima [10], Mirounga leonina [83], Monachus monachus [84], Nanophoca vitulinoides [9], Noriphoca gaudini (this study), Ommatophoca rossii [81], Otaria byronia [85], Phoca vitulina [79], Piscophoca pacifica [8], Pliophoca etrusca [6], Pteronarctos goedertae [54] and Thalassoleon mexicanus [55]. Extinct taxa are indicated by a dagger. Noriphoca gaudini indicated in bold.
Similar articles
- A late surviving Pliocene seal from high latitudes of the North Atlantic realm: the latest monachine seal on the southern margin of the North Sea.
Dewaele L, Lambert O, Louwye S. Dewaele L, et al. PeerJ. 2018 Oct 9;6:e5734. doi: 10.7717/peerj.5734. eCollection 2018. PeerJ. 2018. PMID: 30324020 Free PMC article. - Diversity of late Neogene Monachinae (Carnivora, Phocidae) from the North Atlantic, with the description of two new species.
Dewaele L, Peredo CM, Meyvisch P, Louwye S. Dewaele L, et al. R Soc Open Sci. 2018 Mar 7;5(3):172437. doi: 10.1098/rsos.172437. eCollection 2018 Mar. R Soc Open Sci. 2018. PMID: 29657825 Free PMC article. - Reappraisal of the extinct seal "Phoca" vitulinoides from the Neogene of the North Sea Basin, with bearing on its geological age, phylogenetic affinities, and locomotion.
Dewaele L, Amson E, Lambert O, Louwye S. Dewaele L, et al. PeerJ. 2017 May 16;5:e3316. doi: 10.7717/peerj.3316. eCollection 2017. PeerJ. 2017. PMID: 28533965 Free PMC article. - The 1988 and 2002 phocine distemper virus epidemics in European harbour seals.
Härkönen T, Dietz R, Reijnders P, Teilmann J, Harding K, Hall A, Brasseur S, Siebert U, Goodman SJ, Jepson PD, Dau Rasmussen T, Thompson P. Härkönen T, et al. Dis Aquat Organ. 2006 Jan 30;68(2):115-30. doi: 10.3354/dao068115. Dis Aquat Organ. 2006. PMID: 16532603 Review.
Cited by
- Oldest record of monk seals from the North Pacific and biogeographic implications.
Velez-Juarbe J, Valenzuela-Toro AM. Velez-Juarbe J, et al. Biol Lett. 2019 May 31;15(5):20190108. doi: 10.1098/rsbl.2019.0108. Biol Lett. 2019. PMID: 31064312 Free PMC article. - Evidence for a novel cranial thermoregulatory pathway in thalattosuchian crocodylomorphs.
Young MT, Bowman CIW, Erb A, Schwab JA, Witmer LM, Herrera Y, Brusatte SL. Young MT, et al. PeerJ. 2023 May 2;11:e15353. doi: 10.7717/peerj.15353. eCollection 2023. PeerJ. 2023. PMID: 37151298 Free PMC article. - Evolutionary history of Carnivora (Mammalia, Laurasiatheria) inferred from mitochondrial genomes.
Hassanin A, Veron G, Ropiquet A, Jansen van Vuuren B, Lécu A, Goodman SM, Haider J, Nguyen TT. Hassanin A, et al. PLoS One. 2021 Feb 16;16(2):e0240770. doi: 10.1371/journal.pone.0240770. eCollection 2021. PLoS One. 2021. PMID: 33591975 Free PMC article. - A late surviving Pliocene seal from high latitudes of the North Atlantic realm: the latest monachine seal on the southern margin of the North Sea.
Dewaele L, Lambert O, Louwye S. Dewaele L, et al. PeerJ. 2018 Oct 9;6:e5734. doi: 10.7717/peerj.5734. eCollection 2018. PeerJ. 2018. PMID: 30324020 Free PMC article.
References
- Berta A, Churchill M. 2012. Pinniped taxonomy: review of currently recognized species and subspecies, and evidence used for their description. Mammal Rev. 42, 207–234. (doi:10.1111/j.1365-2907.2011.00193.x) - DOI
- Koretsky IA. 2001. Morphology and systematics of the Miocene Phocinae (Mammalia: Carnivora) from Paratethys and the North Atlantic Region. Geol. Hungarica Series Palaeontol. 54, 1–109.
- Koretsky IA. 2003. New finds of Sarmatian seals (Mammalia, Carnivora, Phocinae) from southern Hungary. In Advances in Vertebrate Paleontology ‘Hen to Panta’: a tribute to Constantin Rădulescu and Petre Mihai Samson (eds Petculescu A, Ştiucă E), pp. 63–70. Bucharest, Romania: Emil Racoviţă Institute of Speleology.
- Deméré TA, Berta A, Adam PJ. 2003. Pinnipedimorph evolutionary biogeography. Bull. Am. Museum Nat. His. 279, 32–76. (doi:10.1206/0003-0090(2003)279<0032:C>2.0.CO;2) - DOI
- Bianucci G, Gatt M, Catanzariti R, Sorbi S, Bonavia CG, Curmi R, Varola A. 2011. Systematics, biostratigraphy and evolutionary pattern of the Oligo-Miocene marine mammals from the Maltese Islands. Geobios 44, 549–585. (doi:10.1016/j.geobios.2011.02.009) - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources