Psychedelics Promote Structural and Functional Neural Plasticity - PubMed (original) (raw)
. 2018 Jun 12;23(11):3170-3182.
doi: 10.1016/j.celrep.2018.05.022.
Alexandra C Greb 1, Lindsay P Cameron 2, Jonathan M Wong 2, Eden V Barragan 2, Paige C Wilson 3, Kyle F Burbach 4, Sina Soltanzadeh Zarandi 1, Alexander Sood 5, Michael R Paddy 3, Whitney C Duim 1, Megan Y Dennis 6, A Kimberley McAllister 7, Kassandra M Ori-McKenney 3, John A Gray 8, David E Olson 9
Affiliations
- PMID: 29898390
- PMCID: PMC6082376
- DOI: 10.1016/j.celrep.2018.05.022
Psychedelics Promote Structural and Functional Neural Plasticity
Calvin Ly et al. Cell Rep. 2018.
Abstract
Atrophy of neurons in the prefrontal cortex (PFC) plays a key role in the pathophysiology of depression and related disorders. The ability to promote both structural and functional plasticity in the PFC has been hypothesized to underlie the fast-acting antidepressant properties of the dissociative anesthetic ketamine. Here, we report that, like ketamine, serotonergic psychedelics are capable of robustly increasing neuritogenesis and/or spinogenesis both in vitro and in vivo. These changes in neuronal structure are accompanied by increased synapse number and function, as measured by fluorescence microscopy and electrophysiology. The structural changes induced by psychedelics appear to result from stimulation of the TrkB, mTOR, and 5-HT2A signaling pathways and could possibly explain the clinical effectiveness of these compounds. Our results underscore the therapeutic potential of psychedelics and, importantly, identify several lead scaffolds for medicinal chemistry efforts focused on developing plasticity-promoting compounds as safe, effective, and fast-acting treatments for depression and related disorders.
Keywords: DMT; LSD; MDMA; depression; ketamine; neural plasticity; noribogaine; psychedelic; spinogenesis; synaptogenesis.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
D.E.O. has submitted a patent application related to this work (PCT/US2017/054277).
Figures
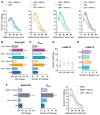
Figure 1. Psychedelics Promote Neuritogenesis both In Vitro and In Vivo
(A) Chemical structures of psychedelics. (B) Representative tracings of cortical neurons (DIV6) treated with compounds. (C) Sholl analysis demonstrates that psychedelics increase dendritic arbor complexity (n = 39–41 neurons). (D) Area under the curve (AUC) of the Sholl plots in (C). (E) Maximum number of crossings (Nmax) of the Sholl plots in (C). (F–I) Cortical neurons treated with psychedelics display an increase in the number of branches (F), the number of primary dendrites (G), and the total length of the dendritic arbor (H) but not the length of the longest dendrite (I). (J and K) Class I neurons from Drosophila larvae treated with psychedelics during the first instar display increased branching (J) but not total length of the dendritic arbor (K) (n = 3 neurons). (L) Representative images of neurons from (J) and (K). (M and N) Class I neurons from Drosophila larvae treated with psychedelics during the third instar display increased branching (M) but not total length of the dendritic arbor (N) (n = 3 neurons). (O) Representative images from (M) and (N). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to vehicle control (VEH). Scale bars, 30 μm. Data are represented as mean ± SEM. See also Figures S1–S5.
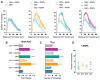
Figure 2. Psychedelics Promote Spinogenesis, Synaptogenesis, and Functional Plasticity
(A) Representative images of cortical neurons (DIV19) treated with compounds for 24 hr, demonstrating that psychedelics increase the number of dendritic spines (blue, MAP2; orange, F-actin). (B) Quantification of spine density (n = 56–65 neurons). (C) Relative proportions of spine types following treatment of cortical cultures with psychedelics (n = 16–21 neurons). (D) Representative images of cortical neurons (DIV19) treated for 24 hr, demonstrating that psychedelics increase synaptogenesis (green, VGLUT1; magenta, PSD-95; yellow, MAP2). White areas in the VGLUT1 + PSD-95 images indicate colocalization of pre- and postsynaptic makers and are indicated by gray arrows. (E–H) Quantification of synapse density (E), synapse size (F), presynaptic density (VGLUT1) (G), and postsynaptic density (PSD-95) (H) following 24-hr treatment of cortical neurons (DIV19) (n = 39–42 neurons). (I) Representative images of Golgi-Cox-stained pyramidal neurons from the PFC of rats 24 hr after receiving a 10 mg/kg dose of DMT. (J) Quantification of spines from (I), demonstrating that DMT (10 mg/kg) increases spinogenesis in vivo to a comparable extent as ketamine (10 mg/kg) (n = 11–17 neurons). (K and L) Whole-cell voltage-clamp recordings of layer V pyramidal neurons from slices obtained 24 hr after DMT treatment (10 mg/kg and 1 mg/kg) demonstrate that DMT increases both spontaneous excitatory postsynaptic current (sEPSC) frequency (K) and amplitude (L) (n = 11–38 neurons from 3 animals). (M) Representative traces for the 10 mg/kg experiments quantified in (K) and (L). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to vehicle control (VEH). Data are represented as mean ± SEM. See also Figure S6.
Figure 3. Psychedelics and BDNF Promote Neuritogenesis via a Related Mechanism
(A–C) Dose response of recombinant BDNF on neuritogenesis. AUC of the Sholl plots (A), Nmax of the Sholl plots (B), and total number of branches (C) of treated cortical neurons (n = 11–12 neurons per treatment, DIV6) indicate that the highest concentration of BDNF (50 ng/mL) is more effective at promoting neuritogenesis than lower concentrations (5.0 and 0.5 ng/mL). (D) Sholl analysis (n = 5–10 neurons) demonstrating that DOI (10 μM) increases neuritogenesis to a comparable extent as recombinant BDNF (50 ng/mL). A combination of DOI (10 μM) and BDNF (50 ng/mL) did not have any additive or synergistic effects. (E) AUC of the Sholl plots in (D). (F) Nmax of the Sholl plots in (D). (G and H) Cultured cortical neurons (DIV18) were treated with compounds for 24 hr, and then BDNF gene (G) and protein (H) expression was assessed via ddPCR (n = 4) and ELISA (n = 3–4), respectively. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to vehicle control (VEH). Data are represented as mean ± SEM.
Figure 4. Psychedelic-Induced Changes in Neuronal Structure Are Mediated by TrkB
(A–D) The effects of psychedelics on dendritic arbor complexity are blocked by ANA-12, a selective inhibitor of TrkB, as measured by Sholl analysis of cultured cortical neurons (A) (DIV6). Compound-induced increases in the AUC of the Sholl plots (B), the Nmax of the Sholl plots (C), and the number of dendritic branches (D) are completely blocked by ANA-12 (n = 8–10 neurons). (E) The spinogenesis-promoting properties of psychedelics are blocked by ANA-12 (n = 19–21 neurons, DIV19). (F) Control experiment demonstrating that ANA-12 blocks the effects of BDNF on neuritogenesis (n = 11–15 neurons). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to vehicle control (VEH) or vehicle + antagonist. Data are represented as mean ± SEM.
Figure 5. Psychedelic-Induced Changes in Neuronal Structure Are Mediated by mTOR
(A–D) The effects of psychedelics on dendritic arbor complexity are blocked by rapamycin, an inhibitor of mTOR, as measured by Sholl analysis of cultured cortical neurons (A) (DIV6). Compound-induced increases in the AUC of the Sholl plots (B), the Nmax of the Sholl plots (C), and the number of dendritic branches (D) are completely blocked by rapamycin (n = 9–12 neurons). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to vehicle control (VEH) or vehicle + antagonist. Data are represented as mean ± SEM.
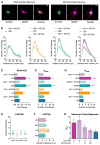
Figure 6. The 5-HT2A Receptor Mediates the Effects of Psychedelics on Structural Plasticity
(A) Rat embryonic cortical neurons express 5-HT2A receptors at both DIV6 and DIV19 (scale bar, 10 μm). (B) The effects of psychedelics on increasing dendritic arbor complexity are blocked by co-treating with ketanserin, a selective antagonist of 5-HT2A receptors, as measured by Sholl analysis of cultured cortical neurons (DIV6). (C–E) Compound-induced increases in the AUC of the Sholl plots (C), the Nmax of the Sholl plots (D), and the number of dendritic branches (E) are completely blocked by ketanserin (n = 10–11 neurons, DIV6). (F) The spinogenesis-promoting properties of psychedelics are blocked by ketanserin (n = 19–20 neurons, DIV19). (G) Ketanserin also blocks the increased Nmax induced by psilocin, noribogaine, and MDMA. (H) Ketanserin dose-dependently blocks the psychoplastogenic effects of 10 nM LSD (n = 9–38 neurons, DIV6). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, as compared to vehicle control (VEH) or vehicle + antagonist. Data are represented as mean ± SEM.
Similar articles
- Psychedelics promote plasticity by directly binding to BDNF receptor TrkB.
Moliner R, Girych M, Brunello CA, Kovaleva V, Biojone C, Enkavi G, Antenucci L, Kot EF, Goncharuk SA, Kaurinkoski K, Kuutti M, Fred SM, Elsilä LV, Sakson S, Cannarozzo C, Diniz CRAF, Seiffert N, Rubiolo A, Haapaniemi H, Meshi E, Nagaeva E, Öhman T, Róg T, Kankuri E, Vilar M, Varjosalo M, Korpi ER, Permi P, Mineev KS, Saarma M, Vattulainen I, Casarotto PC, Castrén E. Moliner R, et al. Nat Neurosci. 2023 Jun;26(6):1032-1041. doi: 10.1038/s41593-023-01316-5. Epub 2023 Jun 5. Nat Neurosci. 2023. PMID: 37280397 Free PMC article. - Biochemical Mechanisms Underlying Psychedelic-Induced Neuroplasticity.
Olson DE. Olson DE. Biochemistry. 2022 Feb 1;61(3):127-136. doi: 10.1021/acs.biochem.1c00812. Epub 2022 Jan 21. Biochemistry. 2022. PMID: 35060714 Free PMC article. Review. - Psychedelics promote neuroplasticity through the activation of intracellular 5-HT2A receptors.
Vargas MV, Dunlap LE, Dong C, Carter SJ, Tombari RJ, Jami SA, Cameron LP, Patel SD, Hennessey JJ, Saeger HN, McCorvy JD, Gray JA, Tian L, Olson DE. Vargas MV, et al. Science. 2023 Feb 17;379(6633):700-706. doi: 10.1126/science.adf0435. Epub 2023 Feb 16. Science. 2023. PMID: 36795823 Free PMC article. - The antidepressant-like effect of alarin is related to TrkB-mTOR signaling and synaptic plasticity.
Zhuang F, Li M, Gao X, Wang Y, Wang D, Ma X, Ma T, Gu S. Zhuang F, et al. Behav Brain Res. 2016 Oct 15;313:158-171. doi: 10.1016/j.bbr.2016.06.057. Epub 2016 Jun 30. Behav Brain Res. 2016. PMID: 27374162 - Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics.
Aleksandrova LR, Phillips AG. Aleksandrova LR, et al. Trends Pharmacol Sci. 2021 Nov;42(11):929-942. doi: 10.1016/j.tips.2021.08.003. Epub 2021 Sep 24. Trends Pharmacol Sci. 2021. PMID: 34565579 Review.
Cited by
- Simulated synapse loss induces depression-like behaviors in deep reinforcement learning.
Chalmers E, Duarte S, Al-Hejji X, Devoe D, Gruber A, McDonald RJ. Chalmers E, et al. Front Comput Neurosci. 2024 Nov 6;18:1466364. doi: 10.3389/fncom.2024.1466364. eCollection 2024. Front Comput Neurosci. 2024. PMID: 39569353 Free PMC article. - Structural neural plasticity evoked by rapid-acting antidepressant interventions.
Liao C, Dua AN, Wojtasiewicz C, Liston C, Kwan AC. Liao C, et al. Nat Rev Neurosci. 2024 Nov 18. doi: 10.1038/s41583-024-00876-0. Online ahead of print. Nat Rev Neurosci. 2024. PMID: 39558048 Review. - Pyramidal cell types and 5-HT2A receptors are essential for psilocybin's lasting drug action.
Shao LX, Liao C, Davoudian PA, Savalia NK, Jiang Q, Wojtasiewicz C, Tan D, Nothnagel JD, Liu RJ, Woodburn SC, Bilash OM, Kim H, Che A, Kwan AC. Shao LX, et al. bioRxiv [Preprint]. 2024 Nov 3:2024.11.02.621692. doi: 10.1101/2024.11.02.621692. bioRxiv. 2024. PMID: 39554087 Free PMC article. Preprint. - Psilocybin-assisted neurofeedback for the improvement of executive functions: a randomized semi-naturalistic-lab feasibility study.
Enriquez-Geppert S, Krc J, O'Higgins FJ, Lietz M. Enriquez-Geppert S, et al. Philos Trans R Soc Lond B Biol Sci. 2024 Dec 2;379(1915):20230095. doi: 10.1098/rstb.2023.0095. Epub 2024 Oct 21. Philos Trans R Soc Lond B Biol Sci. 2024. PMID: 39428872 Clinical Trial. - Psilocybin and the glutamatergic pathway: implications for the treatment of neuropsychiatric diseases.
Szpręgiel I, Bysiek A. Szpręgiel I, et al. Pharmacol Rep. 2024 Dec;76(6):1297-1304. doi: 10.1007/s43440-024-00660-y. Epub 2024 Oct 16. Pharmacol Rep. 2024. PMID: 39412581 Free PMC article. Review.
References
- Alper KR. Ibogaine: a review. Alkaloids Chem Biol. 2001;56:1–38. - PubMed
- Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:353–358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM113770/GM/NIGMS NIH HHS/United States
- T32 MH082174/MH/NIMH NIH HHS/United States
- T32 GM099608/GM/NIGMS NIH HHS/United States
- T32 MH112507/MH/NIMH NIH HHS/United States
- U54 HD079125/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous