Modification of N6-methyladenosine RNA methylation on heat shock protein expression - PubMed (original) (raw)
Modification of N6-methyladenosine RNA methylation on heat shock protein expression
Jiayao Yu et al. PLoS One. 2018.
Abstract
This study was conducted to investigate effect of N6-methyladenosine (m6A) RNA methylation on Heat shock proteins (HSPs) and dissect the profile of HSP RNA methylation. The results showed that m6A methyltransferases METTL3 mRNA was decreased in responses to heat shock stress in HepG2 cells, but m6A-specific binding protein YTHDF2 mRNA was upregulated in a manner similar to HSP70 induction. Immunofluorescence staining showed that the majority of YTHDF2 was present in the cytosol, however, nearly all YTHDF2 translocated from the cytosol into the nucleus after heat shock. METTL3 knockdown significantly changed HSP70, HSP60, and HSP27 mRNA expression in HepG2 cells using siRNA, however, mRNA lifetime was not impacted. Silence of YTHDF2 using siRNA did not change expression of HSP70, but significantly increased HSP90, HSP60, and HSPB1 mRNA expression. In addition, m6A-seq revealed that HSP m6A methylation peaks are mainly enriched on exons and around stop codons, and shows a unique distribution profile in the 5'UTR and 3'UTR. Knockdown of METTL3 changed the methylation patterns of HSPs transcript. In conclusion, m6A RNA methylation regulates HSP gene expression. Differential expression of HSPs modulated by m6A may depend on the m6A site and abundance of the target gene. This finding provides insights into new regulatory mechanisms of HSPs in normal and stress situations.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
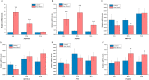
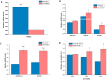
Fig 1. Effect of heat shock on HSPs and m6A mRNA methylation related genes.
Expression of HSPA1B (HSP70) (A), HSPB1 (HSP27) (B), METTL3 (C), METTL14 (D), FTO (E), and YTHDF2 (F) mRNA at 6 h, 12 h, 24 h after heat shock in HepG2 cells. Data are shown as mean ± SEM (n = 3). *p value ≤ 0.05, **p value ≤ 0.01.
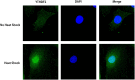
Fig 2. Localization of YTHDF2 under heat shock.
The majority of YTHDF2 resided in the cytosol in normal conditions, whereas nearly all YTHDF2 translocated into the nucleus from the cytosol under heat shock stress. Scale bar = 88 μm.
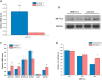
Fig 3. Effect of METTL3 knockdown on HSPs and cell viability in HepG2 cells.
Expression of METTL3 mRNA and protein in HepG2 cells after METTL3 knockdown (A and B). (n = 3). Expression of HSPA1B (HSP70), HSPA9 (HSP70), HSP90AA1 (HSP90), HSPD1 (HSP60), HSF1, and HSPB1 (HSP27) mRNA upon METTL3 knockdown in HepG2 cells (C) (n = 3). The relative cell viability determined by MTT at 24, 48, and 72 h post-transfection of METTL3 siRNA with or without heat shock pretreatment (D) (n = 6). Data are shown as mean ± SEM. *p value ≤ 0.05, **p value ≤ 0.01.
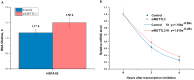
Fig 4. Effect of METTL3 knockdown on the lifetime of HSPA1B in HepG2 cells.
Lifetime of HSPA1B (HSP70) mRNA in the samples following knockdown of METTL3 in HepG2 cells (A). The relative mRNA levels of HSPA1B (HSP70) in the samples following knockdown of METTL3 in HepG2 cells at 0 h, 3 h, and 6 h (B).
Fig 5. Effect of YTHDF2 on HSPs mRNA expression and cell viability in HepG2 cells.
YTHDF2 knockdown decreased YTHDF2 mRNA in HepG2 cells (A). Expression of HSPA1B (HSP70), HSPA9 (HSP70), HSPB1 (HSP27), HSP90AA1 (HSP90), HSPD1 (HSP60) mRNA from the sample of YTHDF2 knockdown in HepG2 cells (B and C). The relative cell viability determined by MTT at 24, 48, and 72 h after knockdown of YTHDF2 with or without heat shock pretreatment (D) (n = 6). Data are shown as mean ± SEM. *p value ≤ 0.05, **p value ≤ 0.01.
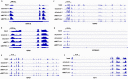
Fig 6. M6A methylated peaks of HSPs mRNA.
Integrative genomics viewer (IGV) plots showing m6A methylated peaks for HSPA1B (HSP70) (A), HSPB1 (HSP27) (B), HSPA9 (HSP70) (C), HSP90AA1 (HSP90) (D), HSPD1 (HSP60) (E), HSF1 (F) mRNA in HepG2 cells. Blue boxes represent exons and blue lines represent introns. n = 2.
Similar articles
- The role of N6-methyladenosine RNA methylation in the heat stress response of sheep (Ovis aries).
Lu Z, Ma Y, Li Q, Liu E, Jin M, Zhang L, Wei C. Lu Z, et al. Cell Stress Chaperones. 2019 Mar;24(2):333-342. doi: 10.1007/s12192-018-00965-x. Epub 2019 Jan 30. Cell Stress Chaperones. 2019. PMID: 30701478 Free PMC article. - Dynamic m(6)A mRNA methylation directs translational control of heat shock response.
Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Zhou J, et al. Nature. 2015 Oct 22;526(7574):591-4. doi: 10.1038/nature15377. Epub 2015 Oct 12. Nature. 2015. PMID: 26458103 Free PMC article. - The Functions of N-methyladenosine (m6A) Modification on HIV-1 mRNA.
Zhong X, Zhou Z, Yang G. Zhong X, et al. Cell Biochem Biophys. 2024 Jun;82(2):561-574. doi: 10.1007/s12013-024-01280-2. Epub 2024 May 16. Cell Biochem Biophys. 2024. PMID: 38753251 Review. - Research advances of N6-methyladenosine in diagnosis and therapy of pancreatic cancer.
Chen S, Ren H, Zhang X, Chang L, Wang Z, Wu H, Zhang J, Ren J, Zhou L. Chen S, et al. J Clin Lab Anal. 2022 Sep;36(9):e24611. doi: 10.1002/jcla.24611. Epub 2022 Jul 15. J Clin Lab Anal. 2022. PMID: 35837987 Free PMC article. Review.
Cited by
- Biogenesis of circRBM33 mediated by N6-methyladenosine and its function in abdominal aortic aneurysm.
Xu Y, Weng X, Qiu J, Wang S. Xu Y, et al. Epigenetics. 2024 Dec;19(1):2392401. doi: 10.1080/15592294.2024.2392401. Epub 2024 Sep 9. Epigenetics. 2024. PMID: 39246182 Free PMC article. - Resveratrol Attenuates High-Fat Diet Induced Hepatic Lipid Homeostasis Disorder and Decreases m6A RNA Methylation.
Wu J, Li Y, Yu J, Gan Z, Wei W, Wang C, Zhang L, Wang T, Zhong X. Wu J, et al. Front Pharmacol. 2020 Dec 18;11:568006. doi: 10.3389/fphar.2020.568006. eCollection 2020. Front Pharmacol. 2020. PMID: 33519432 Free PMC article. - Subcellular relocalization and nuclear redistribution of the RNA methyltransferases TRMT1 and TRMT1L upon neuronal activation.
Jonkhout N, Cruciani S, Santos Vieira HG, Tran J, Liu H, Liu G, Pickford R, Kaczorowski D, Franco GR, Vauti F, Camacho N, Abedini SS, Najmabadi H, Ribas de Pouplana L, Christ D, Schonrock N, Mattick JS, Novoa EM. Jonkhout N, et al. RNA Biol. 2021 Nov;18(11):1905-1919. doi: 10.1080/15476286.2021.1881291. Epub 2021 Feb 15. RNA Biol. 2021. PMID: 33499731 Free PMC article. - HSPA4 upregulation induces immune evasion via ALKBH5/CD58 axis in gastric cancer.
Suo D, Gao X, Chen Q, Zeng T, Zhan J, Li G, Zheng Y, Zhu S, Yun J, Guan XY, Li Y. Suo D, et al. J Exp Clin Cancer Res. 2024 Apr 8;43(1):106. doi: 10.1186/s13046-024-03029-4. J Exp Clin Cancer Res. 2024. PMID: 38589927 Free PMC article.
References
- Oellmy R, Boellmann F. Chaperone regulation of the heat shock protein response Molecular Aspects of the Stress Response: Chaperones, Membranes and Networks: Springer; 2007. p. 89–99. - PubMed
- Zhong X, Li W, Huang X, Zhang L, Yimamu M, Raiput N, et al. Impairment of cellular immunity is associated with overexpression of heat shock protein 70 in neonatal pigs with intrauterine growth retardation. Cell Stress Chaperones. 2012;17(4):495–505. doi: 10.1007/s12192-012-0326-6 - DOI - PMC - PubMed
- Zhong X, Wang T, Zhang X, Li W. Heat shock protein 70 is upregulated in the intestine of intrauterine growth retardation piglets. Cell Stress Chaperones. 2010;15(3):335–42. doi: 10.1007/s12192-009-0148-3 - DOI - PMC - PubMed
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, et al. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones. 2009;14(1):105–11. doi: 10.1007/s12192-008-0068-7 - DOI - PMC - PubMed
- Ciocca DR, Arrigo AP, Calderwood SK. Heat shock proteins and heat shock factor 1 in carcinogenesis and tumor development: an update. Arch Toxicol. 2013;87(1):19–48. doi: 10.1007/s00204-012-0918-z - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by the National Natural Science Foundation of China (31472129) and Natural Science Foundation of Jiangsu province (BK20161446).
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous