In situ architecture of the algal nuclear pore complex - PubMed (original) (raw)
In situ architecture of the algal nuclear pore complex
Shyamal Mosalaganti et al. Nat Commun. 2018.
Abstract
Nuclear pore complexes (NPCs) span the nuclear envelope and mediate nucleocytoplasmic exchange. They are a hallmark of eukaryotes and deeply rooted in the evolutionary origin of cellular compartmentalization. NPCs have an elaborate architecture that has been well studied in vertebrates. Whether this architecture is unique or varies significantly in other eukaryotic kingdoms remains unknown, predominantly due to missing in situ structural data. Here, we report the architecture of the algal NPC from the early branching eukaryote Chlamydomonas reinhardtii and compare it to the human NPC. We find that the inner ring of the Chlamydomonas NPC has an unexpectedly large diameter, and the outer rings exhibit an asymmetric oligomeric state that has not been observed or predicted previously. Our study provides evidence that the NPC is subject to substantial structural variation between species. The divergent and conserved features of NPC architecture provide insights into the evolution of the nucleocytoplasmic transport machinery.
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
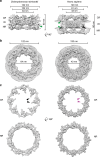
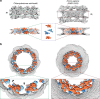
Structure of the _Cr_NPC in comparison to the _Hs_NPC. a Structures are displayed as rendered isosurfaces, sliced through the central axis. Green arrowheads indicate the connector element, which is absent from the cytoplasmic side of the _Cr_NPC. b Cytoplasmic face view. The _Cr_NPC central channel is dilated. c Cytoplasmic and nuclear rings of both NPCs. The _Cr_NPC cytoplasmic ring has reduced density compared to the _Hs_NPC. Black and magenta arrowheads indicate the density attributed to the Nup159 (Nup214 in humans) subcomplex, which forms cytoplasmic filaments that protrude towards the central channel. CR cytoplasmic ring, IR inner ring, and NR nuclear ring
Fig. 2
The inner ring of the _Cr_NPC is dilated compared to the _Hs_NPC. a Structures of the _Cr_NPC and the _Hs_NPC, displayed as rendered isosurfaces, sliced through the central axis. The inner rings (IR) are indicated with dashed boxes (top). The four protomers of the asymmetric unit (orange: outer protomers, blue: inner protomers), each containing Nup93 and Nup62 subcomplexes, explain the inner ring densities of both the _Cr_NPC (bottom left) and _Hs_NPC (bottom right). b View of the _Cr_NPC and _Hs_NPC inner rings seen along the nucleocytoplasmic axis. The asymmetric units (spokes) of the _Cr_NPC inner ring are separated from each other (left), leaving relatively large peripheral channels (arrowheads) between the spokes, whereas the spokes of the _Hs_NPC are positioned closer together (right)
Fig. 3
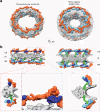
The _Cr_NPC has 24 Y-complexes. a Segmented Y-complexes according to the fits presented in Supplementary Figs. 6, 8, and 9 are shown superimposed with the inner ring structure (gray). The distribution of Y-complexes in the _Cr_NPC is asymmetric across the nuclear envelope plane. The cytoplasmic ring has only eight Y-complexes (orange), whereas the nuclear ring has 16 (orange and light blue). In the _Hs_NPC, the distribution is symmetric, with 16 Y-complexes in both of the outer rings. b Rotated views of the _Cr_NPC and _Hs_NPC, sliced through the central axis. Density attributed to large scaffold Nups (Nup205/Nup188) in the outer rings of the _Hs_NPC (dark blue) is found between the inner (light blue) and outer (orange) copies of the Y-complexes. Similar density is observed in the _Cr_NPC, although this assignment remains tentative at the given resolution. Enlarged views on the bottom row show the presence of only one connector element (green) in the _Cr_NPC (red box), with the cytoplasmic ring lacking the connector (blue box), while the _Hs_NPC contains two connector elements and duplicated Y-complexes (purple box). CR cytoplasmic ring, IR inner ring, and NR nuclear ring
Similar articles
- Architecture of the linker-scaffold in the nuclear pore.
Petrovic S, Samanta D, Perriches T, Bley CJ, Thierbach K, Brown B, Nie S, Mobbs GW, Stevens TA, Liu X, Tomaleri GP, Schaus L, Hoelz A. Petrovic S, et al. Science. 2022 Jun 10;376(6598):eabm9798. doi: 10.1126/science.abm9798. Epub 2022 Jun 10. Science. 2022. PMID: 35679425 Free PMC article. - Architecture of the cytoplasmic face of the nuclear pore.
Bley CJ, Nie S, Mobbs GW, Petrovic S, Gres AT, Liu X, Mukherjee S, Harvey S, Huber FM, Lin DH, Brown B, Tang AW, Rundlet EJ, Correia AR, Chen S, Regmi SG, Stevens TA, Jette CA, Dasso M, Patke A, Palazzo AF, Kossiakoff AA, Hoelz A. Bley CJ, et al. Science. 2022 Jun 10;376(6598):eabm9129. doi: 10.1126/science.abm9129. Epub 2022 Jun 10. Science. 2022. PMID: 35679405 Free PMC article. - Interactome Mapping Reveals the Evolutionary History of the Nuclear Pore Complex.
Obado SO, Brillantes M, Uryu K, Zhang W, Ketaren NE, Chait BT, Field MC, Rout MP. Obado SO, et al. PLoS Biol. 2016 Feb 18;14(2):e1002365. doi: 10.1371/journal.pbio.1002365. eCollection 2016 Feb. PLoS Biol. 2016. PMID: 26891179 Free PMC article. - Functional architecture of the nuclear pore complex.
Grossman E, Medalia O, Zwerger M. Grossman E, et al. Annu Rev Biophys. 2012;41:557-84. doi: 10.1146/annurev-biophys-050511-102328. Annu Rev Biophys. 2012. PMID: 22577827 Review. - [Nuclear pores: from yeast to higher eukaryotes].
Doye V. Doye V. J Soc Biol. 2002;196(4):349-54. J Soc Biol. 2002. PMID: 12645306 Review. French.
Cited by
- Nuclear Envelope Proteins Modulating the Heterochromatin Formation and Functions in Fission Yeast.
Hirano Y, Asakawa H, Sakuno T, Haraguchi T, Hiraoka Y. Hirano Y, et al. Cells. 2020 Aug 16;9(8):1908. doi: 10.3390/cells9081908. Cells. 2020. PMID: 32824370 Free PMC article. Review. - Electron microscopy for imaging organelles in plants and algae.
Weiner E, Pinskey JM, Nicastro D, Otegui MS. Weiner E, et al. Plant Physiol. 2022 Feb 4;188(2):713-725. doi: 10.1093/plphys/kiab449. Plant Physiol. 2022. PMID: 35235662 Free PMC article. - Comprehensive structure and functional adaptations of the yeast nuclear pore complex.
Akey CW, Singh D, Ouch C, Echeverria I, Nudelman I, Varberg JM, Yu Z, Fang F, Shi Y, Wang J, Salzberg D, Song K, Xu C, Gumbart JC, Suslov S, Unruh J, Jaspersen SL, Chait BT, Sali A, Fernandez-Martinez J, Ludtke SJ, Villa E, Rout MP. Akey CW, et al. Cell. 2022 Jan 20;185(2):361-378.e25. doi: 10.1016/j.cell.2021.12.015. Epub 2022 Jan 3. Cell. 2022. PMID: 34982960 Free PMC article. - Breaking the Y.
Holzer G, Antonin W. Holzer G, et al. PLoS Genet. 2019 May 23;15(5):e1008109. doi: 10.1371/journal.pgen.1008109. eCollection 2019 May. PLoS Genet. 2019. PMID: 31120884 Free PMC article. No abstract available. - Evolutionary, structural and functional insights in nuclear organisation and nucleocytoplasmic transport in trypanosomes.
Padilla-Mejia NE, Field MC. Padilla-Mejia NE, et al. FEBS Lett. 2023 Oct;597(20):2501-2518. doi: 10.1002/1873-3468.14747. Epub 2023 Oct 15. FEBS Lett. 2023. PMID: 37789516 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources