Intravenous Epoetin Alfa-epbx versus Epoetin Alfa for Treatment of Anemia in End-Stage Kidney Disease - PubMed (original) (raw)
Randomized Controlled Trial
. 2018 Aug 7;13(8):1204-1214.
doi: 10.2215/CJN.11631017. Epub 2018 Jun 19.
Affiliations
- PMID: 29921734
- PMCID: PMC6086700
- DOI: 10.2215/CJN.11631017
Randomized Controlled Trial
Intravenous Epoetin Alfa-epbx versus Epoetin Alfa for Treatment of Anemia in End-Stage Kidney Disease
Steven Fishbane et al. Clin J Am Soc Nephrol. 2018.
Abstract
Background and objectives: This study was conducted to compare the safety and efficacy of intravenous epoetin alfa-epbx, an epoetin alfa biosimilar, to epoetin alfa in patients on hemodialysis with ESKD and anemia.
Design, setting, participants, & measurements: In this 24-week, multicenter, double-blind comparative efficacy and safety study, 612 patients on hemodialysis with ESKD and anemia who had stable hemoglobin and were receiving stable doses of intravenous epoetin alfa were randomized (1:1) to intravenous epoetin alfa or epoetin alfa-epbx. Dosing was adjusted according to the epoetin alfa prescribing information. The coprimary efficacy end points were the least squares mean difference between the two treatments in mean weekly hemoglobin level and mean weekly epoetin dose per kilogram of body weight during the last 4 weeks of treatment.
Results: The least squares mean difference between epoetin alfa-epbx and epoetin alfa in weekly hemoglobin was -0.12 g/dl and the 95% confidence interval (-0.25 to 0.01) was contained within the prespecified equivalence margin (-0.5 to 0.5 g/dl). The least squares mean difference between epoetin alfa-epbx and epoetin alfa in weekly epoetin dose per kilogram of body weight was 0.37 U/kg per week, and the 95% confidence interval (-10.40 to 11.13) was contained within the prespecified equivalence margin (-45 to 45 U/kg per week). Incidences of adverse events (77.1% versus 75.3%), serious adverse events (24.9% versus 27.0%), and deaths (_n_=5 versus 6) were similar between the epoetin alfa-epbx and epoetin alfa groups, respectively. Five patients tested positive for anti-recombinant human erythropoietin antibodies at baseline, and two additional patients (_n_=1 per group) developed anti-recombinant human erythropoietin antibodies while on study treatment. All patients tested negative for neutralizing antibodies, and no patient in either group experienced an event of pure red cell aplasia.
Conclusions: This 24-week, comparative, clinical trial in patients on hemodialysis with ESKD and anemia demonstrated there is no clinically meaningful difference in efficacy or safety between epoetin alfa-epbx and epoetin alfa.
Keywords: Antibodies, Neutralizing; Biosimilar Pharmaceuticals; Body Weight; Confidence Intervals; Double-Blind Method; EPO protein, human; Epoetin Alfa; Hemoglobins; Incidence; Kidney Failure, Chronic; Least-Squares Analysis; Red-Cell Aplasia, Pure; anemia; chronic kidney disease; erythropoietin; renal dialysis.
Copyright © 2018 by the American Society of Nephrology.
Figures
Graphical abstract
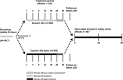
Figure 1.
This comparative clinical trial of IV epoetin alfa-epbx versus epoetin alfa was a randomized, active-controlled, parallel-group, double-blind study including a screening period, 24-week double-blind treatment period, follow-up visit, and 48-week, open-label long-term safety study with epoetin alfa-epbx. aPatients who discontinued early from epoetin alfa-epbx or epoetin alfa received a nonstudy drug standard-of-care ESA until either the follow-up visit or entry into the open-label, long-term safety study. bPatients who completed the treatment period and who did not enter the open-label, long-term safety study underwent a follow-up visit at week 28, 4 weeks after the end of week 24 study activity assessments. cPatients had up to 28 days from completion of the week 24 visit to enter into the open-label, long-term safety study and be treated with IV epoetin alfa-epbx for an additional ≤48 weeks. dCoprimary efficacy end points were assessed during the last 4 weeks of the treatment period.
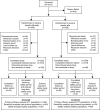
Figure 2.
This flow diagram shows patient disposition through each phase of the comparative clinical trial of IV epoetin alfa-epbx versus epoetin alfa.
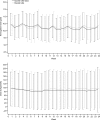
Figure 3.
Mean (SD) weekly hemoglobin level (g/dl) and mean (SD) weekly epoetin dose by body weight (U/kg per week) were similar between epoetin alfa-epbx and epoetin alfa over the duration of the treatment period. ITT population. There was no statistical difference in mean weekly hemoglobin levels (_P_=0.76) or mean weekly epoetin dose per kilogram of body weight (_P_=0.11) between the two treatment groups over 24 weeks.
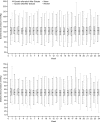
Figure 4.
Mean and median systolic and diastolic BPs after dialysis were unchanged over time and similar between epoetin alfa-epbx and epoetin alfa. The box represents the 25th and 75th percentiles and the tails represent the minimum and maximum observed values; the median is indicated by the horizontal line, and the mean by *.
Similar articles
- Switching from Epoetin Alfa (Epogen®) to Epoetin Alfa-Epbx (RetacritTM) Using a Specified Dosing Algorithm: A Randomized, Non-Inferiority Study in Adults on Hemodialysis.
Thadhani R, Guilatco R, Hymes J, Maddux FW, Ahuja A. Thadhani R, et al. Am J Nephrol. 2018;48(3):214-224. doi: 10.1159/000492621. Epub 2018 Sep 7. Am J Nephrol. 2018. PMID: 30196301 Free PMC article. Clinical Trial. - Randomized Controlled Trial of Subcutaneous Epoetin Alfa-epbx Versus Epoetin Alfa in End-Stage Kidney Disease.
Fishbane S, Spinowitz BS, Wisemandle WA, Martin NE. Fishbane S, et al. Kidney Int Rep. 2019 May 22;4(9):1235-1247. doi: 10.1016/j.ekir.2019.05.010. eCollection 2019 Sep. Kidney Int Rep. 2019. PMID: 31517143 Free PMC article. - Roxadustat (FG-4592) Versus Epoetin Alfa for Anemia in Patients Receiving Maintenance Hemodialysis: A Phase 2, Randomized, 6- to 19-Week, Open-Label, Active-Comparator, Dose-Ranging, Safety and Exploratory Efficacy Study.
Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, Poole L, Saikali KG, Saha G, Hemmerich S, Szczech L, Yu KH, Neff TB. Provenzano R, et al. Am J Kidney Dis. 2016 Jun;67(6):912-24. doi: 10.1053/j.ajkd.2015.12.020. Epub 2016 Feb 2. Am J Kidney Dis. 2016. PMID: 26846333 Clinical Trial. - Short-acting erythropoiesis-stimulating agents for anaemia in predialysis patients.
Hahn D, Esezobor CI, Elserafy N, Webster AC, Hodson EM. Hahn D, et al. Cochrane Database Syst Rev. 2017 Jan 9;1(1):CD011690. doi: 10.1002/14651858.CD011690.pub2. Cochrane Database Syst Rev. 2017. PMID: 28066881 Free PMC article. Review. - Epoetin alfa-epbx: a new entrant into a crowded market. a historical review of the role of erythropoietin stimulating agents and the development of the first epoetin biosimilar in the United States.
Anand S, Al-Mondhiry J, Fischer K, Glaspy J. Anand S, et al. Expert Rev Clin Pharmacol. 2021 Jan;14(1):1-8. doi: 10.1080/17512433.2021.1863786. Epub 2020 Dec 27. Expert Rev Clin Pharmacol. 2021. PMID: 33307871 Review.
Cited by
- Relationship of hemoglobin levels with outcomes in deceased donor kidney transplant: a retrospective cohort study.
Silva BM, Macedo FH, Hayano EEM, Germano S, Ribeiro IF, Franco CA, Requião L, Medina-Pestana J, Goes MA. Silva BM, et al. J Bras Nefrol. 2024 Apr-Jun;46(2):e20230014. doi: 10.1590/2175-8239-JBN-2023-0014en. J Bras Nefrol. 2024. PMID: 38284551 Free PMC article. - Safety outcomes when switching between biosimilars and reference biologics: A systematic review and meta-analysis.
Herndon TM, Ausin C, Brahme NN, Schrieber SJ, Luo M, Andrada FC, Kim C, Sun W, Zhou L, Grosser S, Yim S, Ricci MS. Herndon TM, et al. PLoS One. 2023 Oct 3;18(10):e0292231. doi: 10.1371/journal.pone.0292231. eCollection 2023. PLoS One. 2023. PMID: 37788264 Free PMC article. - Satisfying QTPP of Erythropoietin Biosimilar by QbD through DoE-Derived Downstream Process Engineering.
Nag K, Sarker EH, Kumar S, Chakraborty S, Khan MR, Chowdhury MR, Roy R, Roy R, Biswas BK, Bappi EH, Mohiuddin M, Sultana N. Nag K, et al. Pharmaceutics. 2023 Aug 4;15(8):2087. doi: 10.3390/pharmaceutics15082087. Pharmaceutics. 2023. PMID: 37631301 Free PMC article. - Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis.
Chung EY, Palmer SC, Saglimbene VM, Craig JC, Tonelli M, Strippoli GF. Chung EY, et al. Cochrane Database Syst Rev. 2023 Feb 13;2(2):CD010590. doi: 10.1002/14651858.CD010590.pub3. Cochrane Database Syst Rev. 2023. PMID: 36791280 Free PMC article. Review. - Erythropoietin Mitigates Diabetic Nephropathy by Restoring PINK1/Parkin-Mediated Mitophagy.
Yi X, Yan W, Guo T, Liu N, Wang Z, Shang J, Wei X, Cui X, Sun Y, Ren S, Chen L. Yi X, et al. Front Pharmacol. 2022 May 17;13:883057. doi: 10.3389/fphar.2022.883057. eCollection 2022. Front Pharmacol. 2022. PMID: 35656290 Free PMC article.
References
- Zadrazil J, Horak P: Pathophysiology of anemia in chronic kidney diseases: A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 159: 197–202, 2015 - PubMed
- Kausz AT, Khan SS, Abichandani R, Kazmi WH, Obrador GT, Ruthazer R, Pereira BJ: Management of patients with chronic renal insufficiency in the Northeastern United States. J Am Soc Nephrol 12: 1501–1507, 2001 - PubMed
- Kazmi WH, Kausz AT, Khan S, Abichandani R, Ruthazer R, Obrador GT, Pereira BJ: Anemia: An early complication of chronic renal insufficiency. Am J Kidney Dis 38: 803–812, 2001 - PubMed
- Janssen Products LP: Procrit (Epoetin Alfa) US Prescribing Information. Available at: http://www.janssenlabels.com/package-insert/product-monograph/prescribin.... Accessed February 6, 2018
- Amgen Inc: Epogen (Epoetin Alfa) US Prescribing Information. Available at: https://pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/epogen/e.... Accessed April 17, 2017
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials