Arginase I mRNA therapy - a novel approach to rescue arginase 1 enzyme deficiency - PubMed (original) (raw)
Arginase I mRNA therapy - a novel approach to rescue arginase 1 enzyme deficiency
Kirtika H Asrani et al. RNA Biol. 2018.
Abstract
Arginase I (ARG1) deficiency is an autosomal recessive urea cycle disorder, caused by deficiency of the enzyme Arginase I, resulting in accumulation of arginine in blood. Current Standard of Care (SOC) for ARG1 deficiency in patients or those having detrimental mutations of ARG1 gene is diet control. Despite diet and drug therapy with nitrogen scavengers, ~25% of patients suffer from severe mental deficits and loss of ambulation. 75% of patients whose symptoms can be managed through diet therapy continue to suffer neuro-cognitive deficits. In our research, we demonstrate in vitro and in vivo that administration of ARG1 mRNA increased ARG1 protein expression and specific activity in relevant cell types, including ARG1-deficient patient cell lines, as well as in wild type mice for up to 4 days. These studies demonstrate that ARG1 mRNA treatment led to increased functional protein expression of ARG1 and subsequently an increase in urea. Hence, ARG1 mRNA therapy could be a potential treatment option to develop for patients.
Keywords: Arginase 1; mRNA therapy; urea cycle disorder.
Figures
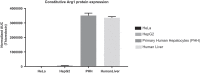
Figure 1.
qPCR analysis for constitutive Arg1 protein expression in four different cell lines. HeLa and HepG2 cells express low levels of Arg1 protein, while primary human hepatocytes (PHH) and human liver sample express high levels of Arg1 protein.
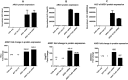
Figure 2.
Arg1 protein expression profile across multiple relevant cell types in upper panel and fold change of protein expression when normalized to eGFP vehicle control in lower panel. A. HeLa, B. HepG2 and C. GM00954 (Arg1 deficient patient fibroblasts) show increased protein expression when transfected with Arg1 mRNA (WT and FLAG).
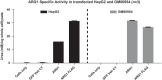
Figure 3.
Specific activity of Arg1 measured in terms of urea synthesized in A. HepG2 and B. GM00954 (Arg1 deficient patient fibroblasts). Note cells only and GFP vehicle controls do not show any urea synthesized due to lack of Arg1 enzyme; when cells are transfected with Arg1 mRNA (WT and FLAG), urea synthesis is increased.
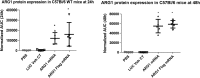
Figure 4.
LNP formulated Arg1 mRNA (WT and FLAG) expresses protein in mouse liver. Arg1 protein expression increases statistically in treated animals at 24h (A) and 48h (B).
Figure 5.
Arg1 immunostaining showed protein biodistribution in hepatocyte cytoplasm, nucleus and sinusoidal spaces at 24h (A) and 48h (B) timepoint in C57Bl/6 wild type mice post Arg1 mRNA treatment (WT and FLAG). 40x magnification.
Figure 6.
In situ hybridization (ISH) to detect Arg1 mRNA in mouse liver treated with Arg1 WT or FLAG-tagged mRNA. Arg1 mRNA detected in hepatocyte cytoplasm (single red dots) and as mRNA clusters (larger red dots) in sinusoidal spaces (40x magnification).
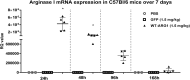
Figure 7.
Arg1 mRNA demonstrated extended duration of protein expression in mouse liver. Arg1 protein expression increases in treated animals at 24h and 48h with lasting protein expression seen up to 168h.
Figure 8.
Extended time course of LNP-formulated Arg1 mRNA (WT) in mouse liver. Upon mRNA treatment, Arg1 mRNA level is high at 24h and is detectable until 7d post-injection.
Similar articles
- Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency.
Truong B, Allegri G, Liu XB, Burke KE, Zhu X, Cederbaum SD, Häberle J, Martini PGV, Lipshutz GS. Truong B, et al. Proc Natl Acad Sci U S A. 2019 Oct 15;116(42):21150-21159. doi: 10.1073/pnas.1906182116. Epub 2019 Sep 9. Proc Natl Acad Sci U S A. 2019. PMID: 31501335 Free PMC article. - Arginase-1 deficiency.
Sin YY, Baron G, Schulze A, Funk CD. Sin YY, et al. J Mol Med (Berl). 2015 Dec;93(12):1287-96. doi: 10.1007/s00109-015-1354-3. Epub 2015 Oct 14. J Mol Med (Berl). 2015. PMID: 26467175 Review. - Inducible arginase 1 deficiency in mice leads to hyperargininemia and altered amino acid metabolism.
Sin YY, Ballantyne LL, Mukherjee K, St Amand T, Kyriakopoulou L, Schulze A, Funk CD. Sin YY, et al. PLoS One. 2013 Nov 4;8(11):e80001. doi: 10.1371/journal.pone.0080001. eCollection 2013. PLoS One. 2013. PMID: 24224027 Free PMC article. - A novel mutation in ARG1 gene is responsible for arginase deficiency in an Asian family.
Hertecant JL, Al-Gazali LI, Karuvantevida NS, Ali BR. Hertecant JL, et al. Saudi Med J. 2009 Dec;30(12):1601-3. Saudi Med J. 2009. PMID: 19936428 - The role and control of arginine levels in arginase 1 deficiency.
Diaz GA, Bechter M, Cederbaum SD. Diaz GA, et al. J Inherit Metab Dis. 2023 Jan;46(1):3-14. doi: 10.1002/jimd.12564. Epub 2022 Oct 13. J Inherit Metab Dis. 2023. PMID: 36175366 Free PMC article. Review.
Cited by
- Nanoparticle-Mediated Mucosal Vaccination: Harnessing Nucleic Acids for Immune Enhancement.
Hussain W, Chaman S, Koser HN, Aun SM, Bibi Z, Pirzadi AN, Hussain J, Zubaria Z, Nabi G, Ullah MW, Wang S, Perveen I. Hussain W, et al. Curr Microbiol. 2024 Jul 20;81(9):279. doi: 10.1007/s00284-024-03803-9. Curr Microbiol. 2024. PMID: 39031239 Review. - Messenger RNA therapy as an option for treating metabolic disorders.
Chandler RJ. Chandler RJ. Proc Natl Acad Sci U S A. 2019 Oct 15;116(42):20804-20806. doi: 10.1073/pnas.1914673116. Epub 2019 Sep 19. Proc Natl Acad Sci U S A. 2019. PMID: 31537746 Free PMC article. No abstract available. - Novel Homozygous Missense Mutation in the ARG1 Gene in a Large Sudanese Family.
Elsayed LEO, Mohammed IN, Hamed AAA, Elseed MA, Salih MAM, Yahia A, Abubaker R, Koko M, Abd Allah ASI, Elbashir MI, Ibrahim ME, Brice A, Ahmed AE, Stevanin G. Elsayed LEO, et al. Front Neurol. 2020 Oct 29;11:569996. doi: 10.3389/fneur.2020.569996. eCollection 2020. Front Neurol. 2020. PMID: 33193012 Free PMC article. - The Pivotal Role of Chemical Modifications in mRNA Therapeutics.
Liu A, Wang X. Liu A, et al. Front Cell Dev Biol. 2022 Jul 13;10:901510. doi: 10.3389/fcell.2022.901510. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35912117 Free PMC article. Review. - mRNA vaccines: A novel weapon to control infectious diseases.
Tian Y, Deng Z, Yang P. Tian Y, et al. Front Microbiol. 2022 Oct 4;13:1008684. doi: 10.3389/fmicb.2022.1008684. eCollection 2022. Front Microbiol. 2022. PMID: 36267192 Free PMC article. Review.
References
- Bk B. Urea cycle disorders. Clin Liver Discov. 2000. November;4(4):815–830. - PubMed
- Helman G, Pacheco-Colon I, Gropman AL. The urea cycle disorders. Semin Neurol. 2014;34(3):341–349. - PubMed
- Carvalho DR, Brum JM, Speck-Martins CE, et al. Clinical features and neurologic progression of hyperargininemia. Pediatr Neurol. 2012;46(6):369–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
This work was supported by the Alexion Pharmaceuticals.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous