Formula versus donor breast milk for feeding preterm or low birth weight infants - PubMed (original) (raw)
Review
Formula versus donor breast milk for feeding preterm or low birth weight infants
Maria Quigley et al. Cochrane Database Syst Rev. 2018.
Update in
- Formula versus donor breast milk for feeding preterm or low birth weight infants.
Quigley M, Embleton ND, McGuire W. Quigley M, et al. Cochrane Database Syst Rev. 2019 Jul 19;7(7):CD002971. doi: 10.1002/14651858.CD002971.pub5. Cochrane Database Syst Rev. 2019. PMID: 31322731 Free PMC article. Updated.
Abstract
Background: When sufficient maternal breast milk is not available, alternative forms of enteral nutrition for preterm or low birth weight (LBW) infants are donor breast milk or artificial formula. Donor breast milk may retain some of the non-nutritive benefits of maternal breast milk for preterm or LBW infants. However, feeding with artificial formula may ensure more consistent delivery of greater amounts of nutrients. Uncertainty exists about the balance of risks and benefits of feeding formula versus donor breast milk for preterm or LBW infants.
Objectives: To determine the effect of feeding with formula compared with donor breast milk on growth and development in preterm or low birth weight (LBW) infants.
Search methods: We used the Cochrane Neonatal search strategy, including electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 6), Ovid MEDLINE, Embase, and the Cumulative Index to Nursing and Allied Health Literature (until 8 June 2017), as well as conference proceedings and previous reviews.
Selection criteria: Randomised or quasi-randomised controlled trials (RCTs) comparing feeding with formula versus donor breast milk in preterm or LBW infants.
Data collection and analysis: Two review authors assessed trial eligibility and risk of bias and extracted data independently. We analysed treatment effects as described in the individual trials and reported risk ratios (RRs) and risk differences (RDs) for dichotomous data, and mean differences (MDs) for continuous data, with respective 95% confidence intervals (CIs). We used a fixed-effect model in meta-analyses and explored potential causes of heterogeneity in subgroup analyses. We assessed the quality of evidence for the main comparison at the outcome level using "Grading of Recommendations Assessment, Development and Evaluation" (GRADE) methods.
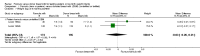
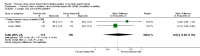
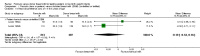
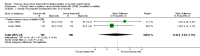
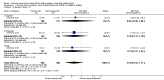
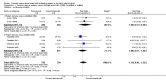
Main results: Eleven trials, in which 1809 infants participated in total, fulfilled the inclusion criteria. Four trials compared standard term formula versus donor breast milk and seven compared nutrient-enriched preterm formula versus donor breast milk. Only the four most recent trials used nutrient-fortified donor breast milk. The trials contain various weaknesses in methodological quality, specifically concerns about allocation concealment in four trials and lack of blinding in most of the trials.Formula-fed infants had higher in-hospital rates of weight gain (mean difference (MD) 2.51, 95% confidence interval (CI) 1.93 to 3.08 g/kg/day), linear growth (MD 1.21, 95% CI 0.77 to 1.65 mm/week) and head growth (MD 0.85, 95% CI 0.47 to 1.23 mm/week). We did not find evidence of an effect on long-term growth or neurodevelopment. Formula feeding increased the risk of necrotising enterocolitis (typical risk ratio (RR) 1.87, 95% CI 1.23 to 2.85; risk difference (RD) 0.03, 95% CI 0.01 to 0.06).The GRADE quality of evidence was moderate for rates of weight gain, linear growth, and head growth (downgraded for high levels of heterogeneity) and was moderate for neurodevelopmental disability, all-cause mortality, and necrotising enterocolitis (downgraded for imprecision).
Authors' conclusions: In preterm and LBW infants, feeding with formula compared with donor breast milk, either as a supplement to maternal expressed breast milk or as a sole diet, results in higher rates of weight gain, linear growth, and head growth and a higher risk of developing necrotising enterocolitis. The trial data do not show an effect on all-cause mortality, or on long-term growth or neurodevelopment.
Conflict of interest statement
MQ: nothing to declare. NDE has conducted research with support from manufacturers of infant formula including Nestec SA (Switzerland), Wyeth UK and Nutricia UK but did not receive any payment, support or benefit in kind for contribution to this review. WM: nothing to declare.
Figures
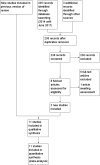
Figure 1
Study flow diagram: 2018 review update.
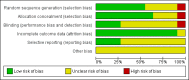
Figure 2
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3
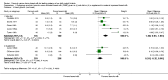
Forest plot of comparison: 1 Formula (term or preterm) versus donor breast milk, outcome: 1.2 Weight gain (g/kg/day).
Figure 4
Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.3 Linear growth (crown‐heel length mm/week).
Figure 5
Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.6 Head growth (mm/week).
Figure 6
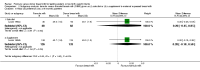
Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.24 All‐cause mortality.
Figure 7
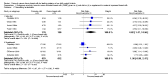
Forest plot of comparison: 1 Formula (term or preterm) versus DBM (unfortified of fortified), outcome: 1.25 Necrotising enterocolitis.
Analysis 1.1
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 1 Time to regain birth weight (days from birth).
Analysis 1.2
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 2 Weight gain (g/kg/day).
Analysis 1.3
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 3 Linear growth (crown‐heel length mm/week).
Analysis 1.4
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 4 Linear growth (crown‐rump length mm/week).
Analysis 1.5
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 5 Linear growth (femoral length mm/week).
Analysis 1.6
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 6 Head growth (mm/week).
Analysis 1.7
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 7 Weight (kg) at 9 months post‐term.
Analysis 1.8
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 8 Length (cm) at 9 months post‐term.
Analysis 1.9
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 9 Head circumference (cm) at 9 months post‐term.
Analysis 1.10
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 10 Weight (kg) at 18 months post‐term.
Analysis 1.11
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 11 Length (cm) at 18 months post‐term.
Analysis 1.12
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 12 Head circumference (cm) at 18 months post‐term.
Analysis 1.13
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 13 Weight (kg) at 7.5 to 8 years of age.
Analysis 1.14
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 14 Length (cm) at 7.5 to 8 years of age.
Analysis 1.15
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 15 Head circumference (cm) at 7.5 to 8 years of age.
Analysis 1.16
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 16 Bayley Mental Development Index at 18 months.
Analysis 1.17
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 17 Bayley Psychomotor Development Index at 18 months.
Analysis 1.18
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 18 Neurodevelopmental disability at 18 months.
Analysis 1.19
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 19 Bayley‐III.
Analysis 1.20
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 20 Bayley‐III score <70.
Analysis 1.21
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 21 Cerebral palsy.
Analysis 1.22
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 22 Hearing impairment.
Analysis 1.23
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 23 Visual impairment.
Analysis 1.24
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 24 All‐cause mortality.
Analysis 1.25
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 25 Necrotising enterocolitis.
Analysis 1.26
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 26 Days after birth to establish full enteral feeding.
Analysis 1.27
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 27 Feeding intolerance or diarrhoea.
Analysis 1.28
Comparison 1 Formula (term or preterm) versus donated breast milk (DBM) (unfortified or fortified), Outcome 28 Incidence of invasive infection.
Analysis 2.1
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 1 Weight gain (g/kg/day).
Analysis 2.2
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 2 Linear grwoth (crown‐heel length mm/week).
Analysis 2.3
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 3 Head growth (mm/week).
Analysis 2.4
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 4 Weight (kg) at 9 months post‐term.
Analysis 2.5
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 5 Length (cm) at 9 months post‐term.
Analysis 2.6
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 6 Head circumference (cm) at 9 months post‐term.
Analysis 2.7
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 7 Weight (kg) at 18 months post‐term.
Analysis 2.8
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 8 Length (cm) at 18 months post‐term.
Analysis 2.9
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 9 Head circumference (cm) at 18 months post‐term.
Analysis 2.10
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 10 Weight (kg) at 7.5 to 8 years of age.
Analysis 2.11
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 11 Length (cm) at 7.5 to 8 years of age.
Analysis 2.12
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 12 Head circumference (cm) at 7.5 to 8 years of age.
Analysis 2.13
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 13 Bayley Mental Development Index at 18 months.
Analysis 2.14
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 14 Bayley Psychomotor Development Index at 18 months.
Analysis 2.15
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 15 Neurodevelopmental disability at 18 months.
Analysis 2.16
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 16 All‐cause mortality.
Analysis 2.17
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 17 Necrotising enterocolitis.
Analysis 2.18
Comparison 2 Subgroup analysis: formula versus donated breast milk (DBM) given as (i) sole diet or (ii) a supplement to maternal expressed breast milk, Outcome 18 Incidence of invasive infection.
Update of
- Formula versus donor breast milk for feeding preterm or low birth weight infants.
Quigley M, McGuire W. Quigley M, et al. Cochrane Database Syst Rev. 2014 Apr 22;(4):CD002971. doi: 10.1002/14651858.CD002971.pub3. Cochrane Database Syst Rev. 2014. PMID: 24752468 Updated. Review.
Comment in
Similar articles
- Formula versus donor breast milk for feeding preterm or low birth weight infants.
Quigley M, Embleton ND, McGuire W. Quigley M, et al. Cochrane Database Syst Rev. 2019 Jul 19;7(7):CD002971. doi: 10.1002/14651858.CD002971.pub5. Cochrane Database Syst Rev. 2019. PMID: 31322731 Free PMC article. Updated. - Formula versus donor breast milk for feeding preterm or low birth weight infants.
Quigley M, McGuire W. Quigley M, et al. Cochrane Database Syst Rev. 2014 Apr 22;(4):CD002971. doi: 10.1002/14651858.CD002971.pub3. Cochrane Database Syst Rev. 2014. PMID: 24752468 Updated. Review. - High versus standard volume enteral feeds to promote growth in preterm or low birth weight infants.
Abiramalatha T, Thomas N, Thanigainathan S. Abiramalatha T, et al. Cochrane Database Syst Rev. 2021 Mar 9;3(3):CD012413. doi: 10.1002/14651858.CD012413.pub3. Cochrane Database Syst Rev. 2021. PMID: 33733486 Free PMC article. - Formula milk versus donor breast milk for feeding preterm or low birth weight infants.
Quigley MA, Henderson G, Anthony MY, McGuire W. Quigley MA, et al. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002971. doi: 10.1002/14651858.CD002971.pub2. Cochrane Database Syst Rev. 2007. PMID: 17943776 Updated. Review. - Nutrient-enriched formula versus standard formula for preterm infants.
Walsh V, Brown JVE, Askie LM, Embleton ND, McGuire W. Walsh V, et al. Cochrane Database Syst Rev. 2019 Jul 17;7(7):CD004204. doi: 10.1002/14651858.CD004204.pub3. Cochrane Database Syst Rev. 2019. PMID: 31314903 Free PMC article.
Cited by
- Nonprofit human milk banking: On a challenging path to global equity.
Shenker NS, Nangia S. Shenker NS, et al. Matern Child Nutr. 2024 Jun;20 Suppl 4(Suppl 4):e13623. doi: 10.1111/mcn.13623. Epub 2024 Jan 10. Matern Child Nutr. 2024. PMID: 38204285 Free PMC article. No abstract available. - Effects of a WeChat Mini-Program on Human Milk Feeding Rates in a Neonatal Intensive Care Unit During the COVID-19 Pandemic.
Jiang C, Chu X, Yu Z, Chen X, Zhang J, Han S. Jiang C, et al. Front Pediatr. 2022 Jun 21;10:888683. doi: 10.3389/fped.2022.888683. eCollection 2022. Front Pediatr. 2022. PMID: 35799691 Free PMC article. - Impact of Donor Human Milk in the Preterm Very Low Birth Weight Gut Transcriptome Profile by Use of Exfoliated Intestinal Cells.
Parra-Llorca A, Gormaz M, Lorente-Pozo S, Cernada M, García-Robles A, Torres-Cuevas I, Kuligoswki J, Collado MC, Serna E, Vento M. Parra-Llorca A, et al. Nutrients. 2019 Nov 5;11(11):2677. doi: 10.3390/nu11112677. Nutrients. 2019. PMID: 31694290 Free PMC article. - Effect of brewer's yeast or beta-glucan on breast milk supply following preterm birth: the BLOOM study - protocol for a multicentre randomised controlled trial.
Grzeskowiak LE, Rumbold AR, Williams L, Kam RL, Ingman WV, Keir A, Martinello KA, Amir LH. Grzeskowiak LE, et al. Int Breastfeed J. 2024 Jun 20;19(1):43. doi: 10.1186/s13006-024-00650-z. Int Breastfeed J. 2024. PMID: 38902831 Free PMC article. - Supplementation-based hypoglycemia guidelines including donor breast milk reduce NICU admission.
Ponnapakkam A, Rees D, Gallup MC, Ahmad KA, Miller D, Fagiana A, Carr NR. Ponnapakkam A, et al. J Perinatol. 2021 Aug;41(8):2088-2094. doi: 10.1038/s41372-021-01069-8. Epub 2021 May 18. J Perinatol. 2021. PMID: 34006969
References
References to studies included in this review
- Corpeleijn WE, Waard M, Christmann V, Goudoever JB, Jansen‐van der Weide MC, Kooi EM, et al. Effect of donor milk on severe infections and mortality in very low‐birth‐weight Infants: the early nutrition study randomized clinical trial. JAMA Pediatrics 2016;170(7):654‐61. [DOI: 10.1001/jamapediatrics.2016.0183; PUBMED: 27135598] - DOI - PubMed
- Cristofalo EA, Schanler RJ, Blanco CL, Sullivan S, Trawoeger R, Kiechl‐Kohlendorfer U, et al. Randomized trial of exclusive human milk versus preterm formula diets in extremely premature infants. Journal of Pediatrics 2013;163(6):1592‐5.e1. [PUBMED: 23968744] - PubMed
- Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet 1990;336(8730):1519‐23. [PUBMED: 1979363] - PubMed
- Lucas A, Gore SM, Cole TJ, Bamford MF, Dossetor JF, Barr I, et al. Multicentre trial on feeding low birthweight infants: effects of diet on early growth. Archives of Disease in Childhood 1984;59(8):722‐30. [PUBMED: 6476868 ] - PMC - PubMed
- Lucas A, Morley R, Cole TJ, Gore SM. A randomised multicentre study of human milk versus formula and later development in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 1994;70(2):F141‐6. [PUBMED: 8154907] - PMC - PubMed
- Lucas A, Morley R, Cole TJ, Gore SM, Davis JA, Bamford MF, et al. Early diet in preterm babies and developmental status in infancy. Archives of Disease in Childhood 1989;64(11):1570‐8. [PUBMED: 2690739] - PMC - PubMed
- Morley R, Lucas A. Randomized diet in the neonatal period and growth performance until 7.5‐8 y of age in preterm children. American Journal of Clinical Nutrition 2000;71(3):822‐8. [DOI: 10.1093/ajcn/71.3.822; PUBMED: 10702179] - DOI - PubMed
References to studies excluded from this review
- Cooper PA, Rothberg AD, Pettifor JM, Bolton KD, Devenhuis S. Growth and biochemical response of premature infants fed pooled preterm milk or special formula. Journal of Pediatric Gastroenterology and Nutrition 1984;3(5):749‐54. [PUBMED: 6502375] - PubMed
- Hair AB, Bergner EM, Lee ML, Moreira AG, Hawthorne KM, Rechtman DJ, et al. Premature infants 750‐1,250 g birth weight supplemented with a novel human milk‐derived cream are discharged sooner. Breastfeeding Medicine 2016;11:133‐7. [DOI: 10.1089/bfm.2015.0166; PUBMED: 26982282] - DOI - PMC - PubMed
- Hair AB, Blanco CL, Moreira AG, Hawthorne KM, Lee ML, Rechtman DJ, et al. Randomized trial of human milk cream as a supplement to standard fortification of an exclusive human milk‐based diet in infants 750‐1250 g birth weight. Journal of Pediatrics 2014;165(5):915‐20. [DOI: 10.1016/j.jpeds.2014.07.005; NCT01487928; PUBMED: 25130571] - DOI - PubMed
- Jarvenpaa AL, Raiha NC, Rassin DK, Gaull GE. Feeding the low‐birth‐weight infant: I. Taurine and cholesterol supplementation of formula does not affect growth and metabolism. Pediatrics 1983;71(2):171‐8. [PUBMED: 6823418] - PubMed
References to studies awaiting assessment
- Calderón Pérez, ML. Effects of feeding with pasteurized human milk compared to standard premature formula in infants less than 2,500 grams. [Efectos de la alimentación con leche humana pasteurizada comparada con fórmula para prematuro estándar en recién nacidos menores de 2,500 gramos] [Masters thesis]. San Carlos, Guatemala: University of San Carlos, 2015. [http://www.repositorio.usac.edu.gt/1907/]
References to ongoing studies
- JPRN‐UMIN000013922. Feeding tolerance of a formula for premature infants versus donor breast milk in the first two weeks of life: a randomized not‐inferiority trial. <apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000013922> (first received 31 December 2014).
- NCT01232725. Donor human milk and neurodevelopmental outcomes in very low birthweight (VLBW) infants [Clinical epidemiologic and biologic studies of donor human milk and breastfeeding]. <clinicaltrials.gov/show/NCT01232725> (first received 02 November 2010).
- NCT01390753. Role of human milk bank in the protection of severe respiratory disease in very low birth weight premature infants [Preventing respiratory disease hospitalizations in premature infants fed donor human milk]. <clinicaltrials.gov/show/NCT01390753> (first received 11 July 2011).
- NCT01534481. Donor milk vs. formula in extremely low birth weight (ELBW) infants [Neurodevelopmental effects of donor human milk vs. preterm formula in extremely low birth weight (ELBW) infants]. <clinicaltrials.gov/show/NCT01534481> (first received 16 February 2012).
- NCT01686477. Preterm formula or donor breast milk for premature babies [Preterm formula or donor breast milk to make up any shortfall in mother's own milk]. <clinicaltrials.gov/show/NCT01686477> (first received 18 September 2012).
Additional references
- Agostoni C, Buonocore G, Carnielli VP, Curtis M, Darmaun D, Decsi T, et al. ESPGHAN Committee on Nutrition. Enteral nutrient supply for preterm infants: commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. Journal of Pediatric Gastroenterology and Nutrition 2010;50(1):85‐91. [DOI: 10.1097/MPG.0b013e3181adaee0; PUBMED: 19881390] - DOI - PubMed
- Amiel‐Tison C, Grenier A. Neurological Assessment During the First Year of Life. Oxford: Oxford University Press, 1986.
- Arslanoglu S, Corpeleijn W, Moro G, Braegger C, Campoy C, Colomb V, et al. ESPGHAN Committee on Nutrition. Donor human milk for preterm infants: current evidence and research directions. Journal of Pediatric Gastroenterology and Nutrition 2013;57(4):535‐42. [DOI: 10.1097/MPG.0b013e3182a3af0a; PUBMED: 24084373] - DOI - PubMed
References to other published versions of this review
- Quigley M, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. The Cochrane Database of Systematic Reviews 2014, (4):CD002971. [PUBMED: 24752468] - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous