Cardiovascular and Renal Outcomes With Canagliflozin According to Baseline Kidney Function - PubMed (original) (raw)
. 2018 Oct 9;138(15):1537-1550.
doi: 10.1161/CIRCULATIONAHA.118.035901.
Toshiaki Ohkuma 1, Bruce Neal 1, David R Matthews 2, Dick de Zeeuw 3, Kenneth W Mahaffey 4, Greg Fulcher 5, Mehul Desai 6, Qiang Li 1, Hsiaowei Deng 6, Norm Rosenthal 6, Meg J Jardine 1 7, George Bakris 8, Vlado Perkovic 1 5
Affiliations
- PMID: 29941478
- PMCID: PMC6181277
- DOI: 10.1161/CIRCULATIONAHA.118.035901
Cardiovascular and Renal Outcomes With Canagliflozin According to Baseline Kidney Function
Brendon L Neuen et al. Circulation. 2018.
Abstract
Background: Canagliflozin is approved for glucose lowering in type 2 diabetes and confers cardiovascular and renal benefits. We sought to assess whether it had benefits in people with chronic kidney disease, including those with an estimated glomerular filtration rate (eGFR) between 30 and 45 mL/min/1.73 m2 in whom the drug is not currently approved for use.
Methods: The CANVAS Program randomized 10 142 participants with type 2 diabetes and eGFR >30 mL/min/1.73 m2 to canagliflozin or placebo. The primary outcome was a composite of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke, with other cardiovascular, renal, and safety outcomes. This secondary analysis describes outcomes in participants with and without chronic kidney disease, defined as eGFR <60 and ≥60 mL/min/1.73 m2, and according to baseline kidney function (eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2).
Results: At baseline, 2039 (20.1%) participants had an eGFR <60 mL/min/1.73 m2, 71.6% of whom had a history of cardiovascular disease. The effect of canagliflozin on the primary outcome was similar in people with chronic kidney disease (hazard ratio, 0.70; 95% CI, 0.55-0.90) and those with preserved kidney function (hazard ratio, 0.92; 95% CI, 0.79-1.07; P heterogeneity = 0.08). Relative effects on most cardiovascular and renal outcomes were similar across eGFR subgroups, with possible heterogeneity suggested only for the outcome of fatal/nonfatal stroke ( P heterogeneity = 0.01), as were results for almost all safety outcomes.
Conclusions: The effects of canagliflozin on cardiovascular and renal outcomes were not modified by baseline level of kidney function in people with type 2 diabetes and a history or high risk of cardiovascular disease down to eGFR levels of 30 mL/min/1.73 m2. Reassessing current limitations on the use of canagliflozin in chronic kidney disease may allow additional individuals to benefit from this therapy.
Clinical trial registration: URL: https://www.clinicaltrials.gov . Unique identifiers: NCT01032629, NCT01989754.
Keywords: canagliflozin; cardiovascular diseases; diabetes mellitus, type 2; glomerular filtration rate; kidney; renal insufficiency, chronic; sodium glucose cotransporter 2; treatment outcome.
Figures
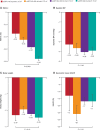
Figure 1.
Changes in intermediate outcomes with canagliflozin compared to placebo in participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. Represents the mean difference in change from baseline between canagliflozin and placebo from post-baseline to end of follow-up, except for UACR, where it is percent change in the geometric mean of canagliflozin relative to placebo. BP indicates blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycohemoglobin; and UACR, urinary albumin/creatinine ratio.
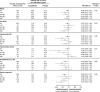
Figure 2.
Effects of canagliflozin on cardiovascular and renal outcomes in participants according to baseline eGFR categories <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2. CV indicates cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiovascular event; and MI, myocardial infarction. *Renal composite: 40% decrease in eGFR, end-stage kidney disease, or renal death.
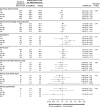
Figure 3.
Effect on eGFR slope from week 6/13 until end of follow-up in participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. eGFR indicates estimated glomerular filtration rate; and SE, standard error. *Data are mean±SE. †Data are reported for week 6 in CANVAS and week 13 in CANVAS-R.
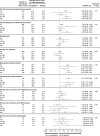
Figure 4.
Adverse events across the CANVAS Program in participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. eGFR indicates estimated glomerular filtration rate; and HR, hazard ratio.
Figure 6.
Absolute benefits and risks per 1000 patients over 5 years with canagliflozin versus placebo in the overall population and in participants with eGFR <45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. eGFR indicates estimated glomerular filtration rate; HF, heart failure; and MACE, major adverse cardiovascular event. *Excess number is relative to the placebo group. If the number is negative, then fewer participants in the canagliflozin group experienced the event compared to the placebo group. †Renal composite: 40% decrease in eGFR, end-stage kidney disease, or renal death.
Figure 5.
Adverse events collected in CANVAS alone in participants with eGFR ≤45, 45 to <60, 60 to <90, and ≥90 mL/min/1.73 m2 at baseline. The annualized incidence rates, estimates for HRs, and 95% CIs are reported for the CANVAS study alone through January 7, 2014, because after this time, only serious adverse events or adverse events leading to study drug discontinuation, or selected adverse events of interest were collected. eGFR indicates estimated glomerular filtration rate; and HR, hazard ratio. *Note that 1 patient in the placebo group who experienced an event had a missing baseline eGFR value; therefore, this patient is only counted in the overall total. †Data collected in CANVAS and CANVAS-R. Includes infections of male genitalia and phimosis and excludes circumcision.
Comment in
- In high-risk T2DM, canagliflozin reduced CV events regardless of baseline renal function.
Herrington WG, Zhu D, Haynes R. Herrington WG, et al. Ann Intern Med. 2019 Feb 19;170(4):JC15. doi: 10.7326/ACPJ201902190-015. Ann Intern Med. 2019. PMID: 30776804 No abstract available.
Similar articles
- Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study).
Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Fabbrini E, Sun T, Li Q, Desai M, Matthews DR; CANVAS Program Collaborative Group. Mahaffey KW, et al. Circulation. 2018 Jan 23;137(4):323-334. doi: 10.1161/CIRCULATIONAHA.117.032038. Epub 2017 Nov 13. Circulation. 2018. PMID: 29133604 Free PMC article. Clinical Trial. - Empagliflozin and Clinical Outcomes in Patients With Type 2 Diabetes Mellitus, Established Cardiovascular Disease, and Chronic Kidney Disease.
Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, Woerle HJ, Broedl UC, von Eynatten M, Zinman B; EMPA-REG OUTCOME Investigators. Wanner C, et al. Circulation. 2018 Jan 9;137(2):119-129. doi: 10.1161/CIRCULATIONAHA.117.028268. Epub 2017 Sep 13. Circulation. 2018. PMID: 28904068 Clinical Trial. - Canagliflozin and renal outcomes in type 2 diabetes: results from the CANVAS Program randomised clinical trials.
Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Erondu N, Shaw W, Barrett TD, Weidner-Wells M, Deng H, Matthews DR, Neal B. Perkovic V, et al. Lancet Diabetes Endocrinol. 2018 Sep;6(9):691-704. doi: 10.1016/S2213-8587(18)30141-4. Epub 2018 Jun 21. Lancet Diabetes Endocrinol. 2018. PMID: 29937267 Clinical Trial. - Class effects of SGLT2 inhibitors on cardiorenal outcomes.
Kluger AY, Tecson KM, Lee AY, Lerma EV, Rangaswami J, Lepor NE, Cobble ME, McCullough PA. Kluger AY, et al. Cardiovasc Diabetol. 2019 Aug 5;18(1):99. doi: 10.1186/s12933-019-0903-4. Cardiovasc Diabetol. 2019. PMID: 31382965 Free PMC article. Review. - The Effects of SGLT2 Inhibitors on Cardiovascular and Renal Outcomes in Diabetic Patients: A Systematic Review and Meta-Analysis.
Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, McCullough PA, Rangaswami J. Lo KB, et al. Cardiorenal Med. 2020;10(1):1-10. doi: 10.1159/000503919. Epub 2019 Nov 19. Cardiorenal Med. 2020. PMID: 31743918
Cited by
- Sodium-Glucose CoTransporter-2 Inhibitor Empagliflozin Ameliorates Sunitinib-Induced Cardiac Dysfunction via Regulation of AMPK-mTOR Signaling Pathway-Mediated Autophagy.
Ren C, Sun K, Zhang Y, Hu Y, Hu B, Zhao J, He Z, Ding R, Wang W, Liang C. Ren C, et al. Front Pharmacol. 2021 Apr 29;12:664181. doi: 10.3389/fphar.2021.664181. eCollection 2021. Front Pharmacol. 2021. PMID: 33995090 Free PMC article. - SGLT2 Inhibition for CKD and Cardiovascular Disease in Type 2 Diabetes: Report of a Scientific Workshop Sponsored by the National Kidney Foundation.
Tuttle KR, Brosius FC 3rd, Cavender MA, Fioretto P, Fowler KJ, Heerspink HJL, Manley T, McGuire DK, Molitch ME, Mottl AK, Perreault L, Rosas SE, Rossing P, Sola L, Vallon V, Wanner C, Perkovic V. Tuttle KR, et al. Diabetes. 2021 Jan;70(1):1-16. doi: 10.2337/dbi20-0040. Epub 2020 Oct 26. Diabetes. 2021. PMID: 33106255 Free PMC article. Review. - SGLT2 Inhibitors and Kidney and Cardiac Outcomes According to Estimated GFR and Albuminuria Levels: A Meta-analysis of Randomized Controlled Trials.
Chun KJ, Jung HH. Chun KJ, et al. Kidney Med. 2021 Jun 19;3(5):732-744.e1. doi: 10.1016/j.xkme.2021.04.009. eCollection 2021 Sep-Oct. Kidney Med. 2021. PMID: 34746739 Free PMC article. - Sodium glucose co-transporter 2 inhibition: a new avenue to protect the kidney.
Heerspink HJL. Heerspink HJL. Nephrol Dial Transplant. 2019 Dec 1;34(12):2015-2017. doi: 10.1093/ndt/gfz033. Nephrol Dial Transplant. 2019. PMID: 30789206 Free PMC article. No abstract available. - Identification of subgroups of patients with type 2 diabetes with differences in renal function preservation, comparing patients receiving sodium-glucose co-transporter-2 inhibitors with those receiving dipeptidyl peptidase-4 inhibitors, using a supervised machine-learning algorithm (PROFILE study): A retrospective analysis of a Japanese commercial medical database.
Zhou FL, Watada H, Tajima Y, Berthelot M, Kang D, Esnault C, Shuto Y, Maegawa H, Koya D. Zhou FL, et al. Diabetes Obes Metab. 2019 Aug;21(8):1925-1934. doi: 10.1111/dom.13753. Epub 2019 Jun 3. Diabetes Obes Metab. 2019. PMID: 31050099 Free PMC article.
References
- Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi 10.1016/S0140-6736(10)60484–9. - PMC - PubMed
- Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. - PubMed
- Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR on behalf of the CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi 10.1056/NEJMoa1611925. - PubMed
- Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous