A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV - PubMed (original) (raw)
. 2018 Aug 29;92(18):e00837-18.
doi: 10.1128/JVI.00837-18. Print 2018 Sep 15.
Lei He # 1, Shihui Sun # 1, Hongjie Qiu 1, Wanbo Tai 1 2, Jiawei Chen 2, Jiangfan Li 1, Yuehong Chen 1, Yan Guo 1, Yufei Wang 2, Jian Shang 3, Kaiyuan Ji 4, Ruiwen Fan 4, Enqi Du 5, Shibo Jiang 2, Fang Li # 3, Lanying Du # 6, Yusen Zhou # 7 8
Affiliations
- PMID: 29950421
- PMCID: PMC6146697
- DOI: 10.1128/JVI.00837-18
A Novel Nanobody Targeting Middle East Respiratory Syndrome Coronavirus (MERS-CoV) Receptor-Binding Domain Has Potent Cross-Neutralizing Activity and Protective Efficacy against MERS-CoV
Guangyu Zhao et al. J Virol. 2018.
Abstract
The newly emerged Middle East respiratory syndrome coronavirus (MERS-CoV) continues to infect humans and camels, calling for efficient, cost-effective, and broad-spectrum strategies to control its spread. Nanobodies (Nbs) are single-domain antibodies derived from camelids and sharks and are potentially cost-effective antivirals with small size and great expression yield. In this study, we developed a novel neutralizing Nb (NbMS10) and its human-Fc-fused version (NbMS10-Fc), both of which target the MERS-CoV spike protein receptor-binding domain (RBD). We further tested their receptor-binding affinity, recognizing epitopes, cross-neutralizing activity, half-life, and efficacy against MERS-CoV infection. Both Nbs can be expressed in yeasts with high yield, bind to MERS-CoV RBD with high affinity, and block the binding of MERS-CoV RBD to the MERS-CoV receptor. The binding site of the Nbs on the RBD was mapped to be around residue Asp539, which is part of a conserved conformational epitope at the receptor-binding interface. NbMS10 and NbMS10-Fc maintained strong cross-neutralizing activity against divergent MERS-CoV strains isolated from humans and camels. Particularly, NbMS10-Fc had significantly extended half-life in vivo; a single-dose treatment of NbMS10-Fc exhibited high prophylactic and therapeutic efficacy by completely protecting humanized mice from lethal MERS-CoV challenge. Overall, this study proves the feasibility of producing cost-effective, potent, and broad-spectrum Nbs against MERS-CoV and has produced Nbs with great potentials as anti-MERS-CoV therapeutics.IMPORTANCE Therapeutic development is critical for preventing and treating continual MERS-CoV infections in humans and camels. Because of their small size, nanobodies (Nbs) have advantages as antiviral therapeutics (e.g., high expression yield and robustness for storage and transportation) and also potential limitations (e.g., low antigen-binding affinity and fast renal clearance). Here, we have developed novel Nbs that specifically target the receptor-binding domain (RBD) of MERS-CoV spike protein. They bind to a conserved site on MERS-CoV RBD with high affinity, blocking RBD's binding to MERS-CoV receptor. Through engineering a C-terminal human Fc tag, the in vivo half-life of the Nbs is significantly extended. Moreover, the Nbs can potently cross-neutralize the infections of diverse MERS-CoV strains isolated from humans and camels. The Fc-tagged Nb also completely protects humanized mice from lethal MERS-CoV challenge. Taken together, our study has discovered novel Nbs that hold promise as potent, cost-effective, and broad-spectrum anti-MERS-CoV therapeutic agents.
Keywords: MERS-CoV; cross-neutralization; nanobody; protective efficacy; receptor-binding domain; spike protein.
Copyright © 2018 Zhao et al.
Figures
FIG 1
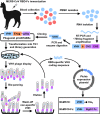
Schematic map for establishment of MERS-CoV Nb library and generation of NbMS10 and NbMS10-Fc Nbs. Blood was collected from MERS-CoV RBD-Fc protein-immunized alpaca after the last immunization to isolate PBMCs. RNA was then extracted to synthesize cDNA via RT-PCR. This was followed by PCR amplification of the N-terminal IgG heavy-chain fragment (∼700 bp), including the VHH gene, while the latter was used as the template to amplify the VHH gene fragment (∼300 to 450 bp). The VHH DNA sequence was further ligated into phagemid vector pCANTAB5e and transformed into E. coli TG1 competent cells to construct VHH library. VHH phage display was carried out to isolate RBD-specific clones. After four rounds of bio-panning, the RBD-specific VHH coding sequence was confirmed from the selected positive clones. The identified VHH coding gene containing a C-terminal His6 or human IgG1 Fc was inserted into Pichia pastoris yeast expression vector pPICZαA to construct NbMS10 and NbMS10-Fc, respectively, for further soluble expression and purification.
FIG 2
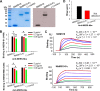
Characterization of MERS-CoV RBD-specific NbMS10 and NbMS10-Fc Nbs. (A) SDS-PAGE and Western blot analyses of purified NbMS10 and NbMS10-Fc. The Nbs were subjected to SDS-PAGE (left) or Western blotting (right), followed by detection using anti-llama antibody. The molecular weight marker (in kDa) is indicated on the left. (B) Detection of binding between NbMS10 or NbMS10-Fc and MERS-CoV S1 (MERS-S1) or RBD (MERS-RBD) protein by ELISA. The plates were coated with MERS-CoV S1-His or RBD-Fd protein (2 μg/ml), followed by sequential incubation with respective Nbs and goat anti-llama and HRP-conjugated anti-goat IgG antibodies. The data are presented as mean _A_450 values ± the standard deviation (SDs) (n = 2). Significant differences (*; **, and ***) are shown in the binding of Nbs to MERS-S1 or MERS-RBD at various concentrations. (C) The binding kinetics between NbMS10 or NbMS10-Fc and MERS-CoV RBD or S1 protein were measured by SPR. MERS-CoV RBD-Fc protein was used for binding to NbMS10 (containing a C-terminal His6), and S1-His protein was used for binding to NbMS10-Fc (containing a C-terminal human Fc). (D) Detection of NbMS10 and NbMS10-Fc neutralizing activity against MERS-CoV infection (EMC2012 strain) by a microneutralization assay. The Nb-MERS-CoV mixtures were incubated with Vero E6 cells and observed for the presence or absence of CPE. Neutralizing activity of Nbs was recorded as the concentration of Nbs in complete inhibition of MERS-CoV-induced CPE in at least 50% of the wells (ND50). The data are expressed as mean ND50 ± the SD (n = 3). The experiments were repeated twice, and similar results were obtained. The “(−) control” in panels A, B, and D refers to SARS-CoV 33G4 mouse MAb.
FIG 3
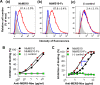
Determination of mechanisms of NbMS10 and NbMS10-Fc Nbs by flow cytometry and ELISA analyses. (A and B) Flow cytometry analysis of NbMS10 and NbMS10-Fc in inhibiting the binding between MERS-CoV RBD and cell-associated hDPP4 receptor. (A) Gray shading indicates the Huh-7 cell control. The red line indicates the binding of MERS-CoV RBD (i.e., RBD-Fc protein, 20 μg/ml) to Huh-7 cells. The blue line indicates NbMS10 (a) and NbMS10-Fc (b) Nbs (10 μg/ml) or the SARS-CoV 33G4 MAb control (c) inhibited RBD binding to Huh-7 cells. The percent inhibition values are shown in each graph. (B) NbMS10 and NbMS10-Fc demonstrated dose-dependent inhibition of the binding between MERS-CoV RBD and cell-associated hDPP4 in Huh-7 cells. The percent inhibition was calculated as the RBD-Huh-7 cell binding in the presence or absence of Nbs according to the following formula: (1 − RBD-Huh-7-Nb/RBD-Huh-7) × 100. (C) ELISA analysis of NbMS10 and NbMS10-Fc in inhibiting the binding between MERS-CoV RBD and soluble hDPP4 protein. The plates were coated with MERS-CoV RBD-Fc protein (2 μg/ml), followed by sequential incubation with serial dilutions of Nbs or hDPP4 protein (2 μg/ml), goat anti-hDPP4, and HRP-conjugated anti-goat IgG antibodies. The percent inhibition was calculated as the RBD-hDPP4 binding in the presence or absence of Nbs according to the following formula: (1 − RBD-hDPP4-Nb/RBD-hDPP4) × 100. A significant difference (***) occurred between NbMS10 and NbMS10-Fc in inhibition of RBD-hDPP4 binding. The “(−) control” in panels B and C refers to SARS-CoV 33G4 MAb. The data are presented as the mean percent inhibition ± the SD (n = 2). The experiments were repeated twice, and similar results were obtained.
FIG 4
NbMS10 and NbMS10-Fc Nbs recognized conformational epitopes and mapping of Nb neutralizing epitope(s). (A) Mapping of the epitope of NbMS10 by ELISA. The plates were coated with RBD-Fc (RBD-WT) or respective mutant RBD proteins containing a C-terminal human Fc (2 μg/ml), followed by sequential incubation with serial dilutions of NbMS10 (containing a C-terminal His6), mouse anti-His and HRP-conjugated anti-mouse IgG antibodies. The data are presented as mean _A_450 values ± the SD (n = 3). (B) Inhibitory effect of NbMS10 and NbMS10-Fc against infection of MERS-CoV pseudoviruses with (MERS-D539A) or without (MERS-WT) D539A mutation. The data are presented as the mean percent inhibition ± the SD (n = 4). (C) Binding of MERS-CoV RBD with (MERS-D539A) or without (MERS-WT) D539A mutation to hDPP4 protein by ELISA. The data are presented as mean _A_450 values ± the SD (n = 4). A significant difference (***) occurred between MERS-WT and MERS-D539A in binding to hDPP4. (D) Detection of the binding between NbMS10 or NbMS10-Fc and MERS-CoV RBD by ELISA in the presence or absence of DTT. The plates were coated with RBD-Fd protein (2 μg/ml) and treated with or without DTT, followed by sequential incubation with serial dilutions of NbMS10 or NbMS10-Fc and goat anti-llama and HRP-conjugated anti-goat IgG antibodies. The data are presented as mean _A_450 values ± the SD (n = 2). The “(−) control” in panels B and D refers to SARS-CoV 33G4 MAb. The above-described experiments were repeated twice, and similar results were obtained.
FIG 5
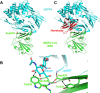
Proposed structural mechanisms for the neutralizing activity of NbMS10 and NbMS10-Fc Nbs. (A) Crystal structure of MERS-CoV RBD complexed with hDPP4 receptor (PDB
4KR0
). MERS-CoV RBD is colored in green, and hDPP4 is colored in cyan. RBD residue Asp539, which is critical for the binding of the Nbs to the RBD, is shown in sticks. (B) Structural role of RBD residue Asp539 at the interface between MERS-CoV RBD and hDPP4 (PDB
4KR0
). RBD residue Asp539 forms a critical salt bridge with DPP4 residue 267, a van der Waals interaction with RBD residue Tyr541, and a hydrogen bond with the main chain nitrogen of RBD residue Glu536. Near Asp539 is an N-linked glycan from DPP4 that forms strong and favorable van der Waals stacking with RBD residue Trp535. Dotted lines indicate hydrogen bonds, and arrows indicate van der Waals interactions. (C) Proposed structural mechanisms for the neutralizing activity of NbMS10 and NbMS10-Fc Nbs. The Nbs (colored in red) bind to the RBD epitope surrounding Asp539, disrupting the binding interactions between the RBD and DPP4 and physically blocking the binding of DPP4 to the RBD.
FIG 6
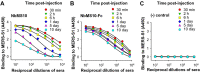
Detection of half-lives of Nbs in C57BL/6 mice. Sera were collected from mice injected with NbMS10 (A), NbMS10-Fc (B), or PBS control (C) at the indicated time points and then tested by ELISA for binding with the MERS-CoV S1 protein. The plates were coated with S1-His protein (2 μg/ml), and the data are presented as mean _A_450 values ± the SD of mice (n = 5) in each group.
FIG 7
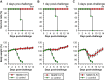
Evaluation of prophylactic and therapeutic efficacy of NbMS10-Fc in hDPP4-Tg mice. The hDPP4-Tg mice were treated with NbMS10-Fc or Trastuzumab “(−) control” (10 mg/kg) 3 days preinfection (A) or 1 day (B) and 3 days (C) postinfection with the MERS-CoV (EMC2012 strain, 105.3 TCID50). Virus-challenged mice were monitored for 14 days to evaluate survival rate (above) and body weight changes (below). The body weight data are presented as means ± the SD of mice in each group (n = 6). Significant differences (** and ***) are indicated between the NbMS10-Fc and control groups.
FIG 8
Conservation of residue D539 at the RBD of MERS-CoV S protein. Schematic structure of RBD and mutations of amino acid (aa) residues at the RBM of RBD among natural MERS-CoV isolates. A total of 482 RBM sequences (residues 484 to 567) derived from natural MERS-CoV isolates were aligned, and residues with natural mutations are shown. Residues in the rectangle frame show the RBM consensus, and the positions of corresponding residues are illustrated. The numbers on the left indicate the counts of MERS-CoV isolates with the identical sequence in the analyzed region.
Similar articles
- Recombinant Receptor-Binding Domains of Multiple Middle East Respiratory Syndrome Coronaviruses (MERS-CoVs) Induce Cross-Neutralizing Antibodies against Divergent Human and Camel MERS-CoVs and Antibody Escape Mutants.
Tai W, Wang Y, Fett CA, Zhao G, Li F, Perlman S, Jiang S, Zhou Y, Du L. Tai W, et al. J Virol. 2016 Dec 16;91(1):e01651-16. doi: 10.1128/JVI.01651-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795425 Free PMC article. - Enhanced Ability of Oligomeric Nanobodies Targeting MERS Coronavirus Receptor-Binding Domain.
He L, Tai W, Li J, Chen Y, Gao Y, Li J, Sun S, Zhou Y, Du L, Zhao G. He L, et al. Viruses. 2019 Feb 19;11(2):166. doi: 10.3390/v11020166. Viruses. 2019. PMID: 30791410 Free PMC article. - A humanized neutralizing antibody against MERS-CoV targeting the receptor-binding domain of the spike protein.
Li Y, Wan Y, Liu P, Zhao J, Lu G, Qi J, Wang Q, Lu X, Wu Y, Liu W, Zhang B, Yuen KY, Perlman S, Gao GF, Yan J. Li Y, et al. Cell Res. 2015 Nov;25(11):1237-49. doi: 10.1038/cr.2015.113. Epub 2015 Sep 22. Cell Res. 2015. PMID: 26391698 Free PMC article. - Advances in MERS-CoV Vaccines and Therapeutics Based on the Receptor-Binding Domain.
Zhou Y, Yang Y, Huang J, Jiang S, Du L. Zhou Y, et al. Viruses. 2019 Jan 14;11(1):60. doi: 10.3390/v11010060. Viruses. 2019. PMID: 30646569 Free PMC article. Review. - Neutralizing Monoclonal Antibodies as Promising Therapeutics against Middle East Respiratory Syndrome Coronavirus Infection.
Han HJ, Liu JW, Yu H, Yu XJ. Han HJ, et al. Viruses. 2018 Nov 30;10(12):680. doi: 10.3390/v10120680. Viruses. 2018. PMID: 30513619 Free PMC article. Review.
Cited by
- Camelid VHHs Fused to Human Fc Fragments Provide Long Term Protection Against Botulinum Neurotoxin A in Mice.
Godakova SA, Noskov AN, Vinogradova ID, Ugriumova GA, Solovyev AI, Esmagambetov IB, Tukhvatulin AI, Logunov DY, Naroditsky BS, Shcheblyakov DV, Gintsburg AL. Godakova SA, et al. Toxins (Basel). 2019 Aug 7;11(8):464. doi: 10.3390/toxins11080464. Toxins (Basel). 2019. PMID: 31394847 Free PMC article. - A Review of Potential Therapeutic Strategies for COVID-19.
Meng J, Li R, Zhang Z, Wang J, Huang Q, Nie D, Fan K, Guo W, Zhao Z, Han Z. Meng J, et al. Viruses. 2022 Oct 25;14(11):2346. doi: 10.3390/v14112346. Viruses. 2022. PMID: 36366444 Free PMC article. Review. - Structure defining of ultrapotent neutralizing nanobodies against MERS-CoV with novel epitopes on receptor binding domain.
Ma S, Zhang D, Wang Q, Zhu L, Wu X, Ye S, Wang Y. Ma S, et al. PLoS Pathog. 2024 Aug 14;20(8):e1012438. doi: 10.1371/journal.ppat.1012438. eCollection 2024 Aug. PLoS Pathog. 2024. PMID: 39141662 Free PMC article. - Dromedary camels as a natural source of neutralizing nanobodies against SARS-CoV-2.
Chouchane L, Grivel JC, Farag EABA, Pavlovski I, Maacha S, Sathappan A, Al-Romaihi HE, Abuaqel SW, Ata MMA, Chouchane AI, Remadi S, Halabi N, Rafii A, Al-Thani MH, Marr N, Subramanian M, Shan J. Chouchane L, et al. JCI Insight. 2021 Mar 8;6(5):e145785. doi: 10.1172/jci.insight.145785. JCI Insight. 2021. PMID: 33529170 Free PMC article. - Nanobodies: an unexplored opportunity to combat COVID-19.
Ezzikouri S, Nourlil J, Tsukiyama-Kohara K, Kohara M, El Ossmani H, Windisch MP, Benjelloun S. Ezzikouri S, et al. J Biomol Struct Dyn. 2022 Apr;40(7):3129-3131. doi: 10.1080/07391102.2020.1845801. Epub 2020 Nov 10. J Biomol Struct Dyn. 2022. PMID: 33172342 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI089728/AI/NIAID NIH HHS/United States
- R01 AI110700/AI/NIAID NIH HHS/United States
- R01 AI139092/AI/NIAID NIH HHS/United States
- R21 AI109094/AI/NIAID NIH HHS/United States
- R01 AI137472/AI/NIAID NIH HHS/United States
- R21 AI128311/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous