Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle - PubMed (original) (raw)
Klotho expression is a prerequisite for proper muscle stem cell function and regeneration of skeletal muscle
Hellen E Ahrens et al. Skelet Muscle. 2018.
Abstract
Background: Klotho is a well-known anti-aging hormone, which serves as a suppressor of aging through a variety of mechanisms. Aging of skeletal muscle is concomitant with a decrease in muscle stem cell function resulting in impaired regeneration.
Methods: Here we investigate the functional role of the anti-aging hormone Klotho for muscle stem cell function after cardiotoxin-induced injury of skeletal muscle using a klotho hypomorphic mouse line, which is characterized by a premature aging phenotype. Furthermore, we perform floating single myofiber cultures with their adjacent muscle stem cells to investigate the interplay between canonical Wnt signaling and Klotho function.
Results: We demonstrate that muscle stem cell numbers are significantly decreased in klotho hypomorphic mice. Furthermore, we show that muscle stem cell function is also severely impaired upon loss of klotho expression, in culture and during regeneration in vivo. Moreover, we demonstrate that addition of recombinant Klotho protein inhibits aberrant excessive Wnt signaling in aged muscle stem cells thereby restoring their functionality.
Conclusions: The anti-aging hormone Klotho counteracts aberrant canonical Wnt signaling in muscle stem cells and might be one of the naturally occurring inhibitors of canonical Wnt signaling in skeletal muscle.
Keywords: Aging; Canonical Wnt signaling, Wnt3a; Klotho; Muscle stem cell; Myogenesis; Regeneration; Skeletal muscle.
Conflict of interest statement
Ethics approval
All animal experiments were performed in accordance with the German Animal Welfare Act and approved by the responsible local authority of Thuringia (TLV), TVA no.: 03-11/14.
Competing interests
The authors declare that they have no competing interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Fig. 1
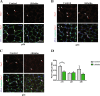
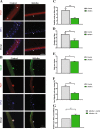
Muscle stem cell numbers are reduced in ΔKlotho mice. a–c Representative immunofluorescence images of TA muscle cross-sections from ΔKlotho and control mice stained for DAPI (DNA, blue) and Laminin (green), and Pax7 (red) at a p56, b p14, and c p21. Scale bar = 50 μm. d Quantification of Pax7-positive SCs on whole TA cross-sections from ΔKlotho and control mice at p14, p21, and p56. n = 3 mice per genotype and time point. All data are presented as means ± SEM. *p < 0.05
Fig. 2
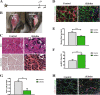
Early regeneration of skeletal muscle is impaired in ΔKlotho mice. a The right TA muscle from ΔKlotho and control mice was injured by injection of cardiotoxin (CTX) at p50 and isolated at p60. b TA muscles (arrows) show extensive fibrosis in ΔKlotho mice 10 days (d10) after injury. c Representative H&E stainings and Alizarin Red stainings of TA muscle cross-sections at d10 after injury. Necrotic tissue, fatty vacuoles, and massive cell invasion are visible in ΔKlotho TA muscles, while control TA muscles show mostly regenerating myofibers. Scale bar = 100 μm. d Immunostainings of cross-sections of TA muscles at d10 after injury with antibodies directed against Laminin (green) and developmental (dev) MHC (red), nuclei are stained with DAPI (DNA, blue). Scale bar = 50 μm. e Minimal fiber feret measured on whole cross-sections from ΔKlotho and control TA muscles at d10 after injury. (ΔKlotho n = 4 mice, control n = 5 mice). Proportion of f devMHC-positive fibers and g Pax7-positive cells per myofiber on whole cross-sections at d10 after CTX-induced injury (ΔKlotho n = 3 mice, control n = 4 mice). h Representative images of stainings for CD68 (in red) and laminin (in green) showing increased infiltration with macrophages in regenerating muscles from ΔKlotho mice. Scale bar = 50 um. All data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 3
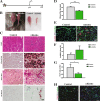
Late regeneration of skeletal muscle is impaired in ΔKlotho mice. a The right TA muscle from ΔKlotho and control mice was injured by injection of cardiotoxin (CTX) at p39 and isolated at p60. b TA muscles (arrows) show signs of impaired regeneration by H&E staining and extensive fibrosis in ΔKlotho mice at day 21 after injury (c), but not in control littermates as demonstrated by Sirius Red staining. Alizarin Red marks calcifications, visible in regenerating muscles from ΔKlotho mice as well as lipid accumulations shown by Oil Red O staining. Scale bar = 100 μm. d Minimal fiber feret measured on whole cross-sections of TA muscles from ΔKlotho and control at d21 after injury. (ΔKlotho n = 4 mice, control n = 4 mice). e Immunostainings of cross-sections of TA muscles at d21 after injury with antibodies directed against laminin (green) and developmental (dev) MHC (red), nuclei are stained with DAPI (DNA, blue). Scale bar = 50 μm. Proportion of (f) devMHC-positive fibers (ΔKlotho n = 2 mice, control n = 4 mice) and (g) Pax7-positive satellite cells per myofiber on whole cross-sections at d21 after CTX-induced injury. (ΔKlotho n = 4 mice, control n = 4 mice). h Representative images of stainings for CD68 (in red) and laminin (in green) showing increased infiltration with macrophages in regenerating muscles from ΔKlotho mice. Scale bar = 50 um. All data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 4
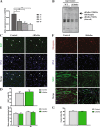
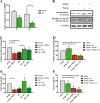
Proliferation and differentiation are not affected in myoblasts from ΔKlotho mice in vitro. a αklotho mRNA expression during myogenic differentiation of myoblasts d0, growth medium (myoblasts); d1, day1 of differentiation (myocytes); d3 and d5, days 3 and 5 of differentiation (myotubes). (n = 3 mice per time point). All data are presented as means ± SEM. *p < 0.05, **p < 0.01. Expression was normalized to GAPDH. b Immunoblot analysis showing expression of sKlotho in the supernatant from primary myoblasts isolated from wt mice but not in the supernatant from ΔKlotho mice. An image of the Ponceau stained membrane can be found in Additional file 3: Figure S3F. c Primary myoblasts from ΔKlotho and control mice were immunostained for the proliferation marker Ki67 (green) and DAPI (DNA, blue) after 48 h of proliferation time in normal culture medium. Arrow heads mark Ki67 positive cells. Scale bar = 100 μm. d The proportion of Ki67-positive cells was counted in 15 randomly chosen regions of interest per condition (ΔKlotho n = 3 mice, control n = 4 mice). e The mean myotube diameter was measured in six randomly chosen regions of interest per condition (ΔKlotho n = 3 mice, control n = 4 mice). f Representative images of immunostainings from myotubes after 5 days of differentiation. DNA (DAPI, blue), MHC (green), myogenin (red). Scale bar = 100 μm. g The fusion index was calculated as the ratio of nuclei in myotubes in relation to the total number of nuclei in 6 randomly chosen regions of interest per condition (ΔKlotho n = 3 mice, control n = 4 mice). All data are presented as means ± SEM
Fig. 5
Muscle stem cell function is impaired in ΔKlotho mice. a Myofibers with their adjacent muscle stem cells were isolated from EDL muscles of p42 old mice and directly fixed and stained with antibodies directed against Pax7 (red) and DNA (DAPI, blue). Arrows point at Pax7-positive muscle stem cells. Scale bar = 50 μm. b Representative images of clusters of muscle stem cells on their adjacent EDL myofibers isolated at p42 and cultured for 72 h and then stained with antibodies directed against MyoD (green), Pax7 (red), and DNA (DAPI, blue). Scale bar = 50 μm. c The number of Pax7-positive muscle stem cells per myofiber was quantified from p42 ΔKlotho and control mice. (ΔKlotho n = 5 mice, control n = 7 mice). d The number of clusters per myofiber isolated from p42 animals and cultured for 72 h of culture was enumerated. (ΔKlotho n = 5 mice, control n = 7 mice). e The number of cells per cluster per myofiber isolated from p42 animals and cultured for 72 h was determined. (ΔKlotho n = 5 mice, control n = 7 mice). f The composition of clusters shows a reduction in the proportion of further differentiated cells (Pax7−/MyoD+) in ΔKlotho compared to control mice at p42. (ΔKlotho n = 5 mice, control n = 7 mice). g Addition of recombinant sKlotho to fiber cultures from ΔKlotho mice results in an increase in cluster number per myofiber (n = 3). All data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 6
sKlotho antagonizes aberrant Wnt3a function in aged muscle stem cells. a αKlotho mRNA expression in myofibers with their adjacent muscle stem cells directly after isolation or after 72 h of culture from young and old C57BL/6J mice (young mice: n = 4, old mice: n = 5). b Addition of sKlotho to primary myoblasts reduces induction of canonical Wnt signaling evoked by addition of recombinant Wnt3a. c Myofibers with their adjacent muscle stem cells from young (4 months) and old (22–24 months) mice were cultured for 72 h with normal medium, recombinant soluble klotho (KL) protein, recombinant Wnt3a, or recombinant Dkk1. (n = 6 mice (young), n = 5 (old)). The number of clusters per myofiber was normalized to young control. d Myofibers with their adjacent muscle stem cells from young (4 months) mice were cultured for 72 h with normal medium, recombinant soluble klotho (KL) protein, recombinant Wnt3a, or a combination of both (n = 6 mice). The number of clusters per myofiber was normalized to young control. e Addition of sKlotho recombinant protein (KL) increases the proportion of Pax7+/MyoD− cells per fiber located in a cluster. The number of Pax7+/MyoD− cells (located in a cluster) per myofiber was normalized to young control (n = 6 mice (young), n = 5 (old)). f Myofibers with their adjacent muscle stem cells from young (4 months) mice were cultured for 72 h with normal medium, recombinant soluble klotho (KL) protein, recombinant Wnt3a, or a combination of both (n = 6 mice). The number of Pax7+/MyoD− cells (located in a cluster) per myofiber was normalized to young control. All data are presented as means ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001
Similar articles
- Klotho, stem cells, and aging.
Bian A, Neyra JA, Zhan M, Hu MC. Bian A, et al. Clin Interv Aging. 2015 Aug 4;10:1233-43. doi: 10.2147/CIA.S84978. eCollection 2015. Clin Interv Aging. 2015. PMID: 26346243 Free PMC article. Review. - Thyroid Hormone Receptor α Plays an Essential Role in Male Skeletal Muscle Myoblast Proliferation, Differentiation, and Response to Injury.
Milanesi A, Lee JW, Kim NH, Liu YY, Yang A, Sedrakyan S, Kahng A, Cervantes V, Tripuraneni N, Cheng SY, Perin L, Brent GA. Milanesi A, et al. Endocrinology. 2016 Jan;157(1):4-15. doi: 10.1210/en.2015-1443. Epub 2015 Oct 9. Endocrinology. 2016. PMID: 26451739 Free PMC article. - Regulation of muscle stem cell function.
von Maltzahn J. von Maltzahn J. Vitam Horm. 2021;116:295-311. doi: 10.1016/bs.vh.2021.02.012. Epub 2021 Mar 9. Vitam Horm. 2021. PMID: 33752822 - Activation of the hypoxia-inducible factor 1α promotes myogenesis through the noncanonical Wnt pathway, leading to hypertrophic myotubes.
Cirillo F, Resmini G, Ghiroldi A, Piccoli M, Bergante S, Tettamanti G, Anastasia L. Cirillo F, et al. FASEB J. 2017 May;31(5):2146-2156. doi: 10.1096/fj.201600878R. Epub 2017 Feb 10. FASEB J. 2017. PMID: 28188178 - Intracellular signaling of the aging suppressor protein Klotho.
Sopjani M, Rinnerthaler M, Kruja J, Dermaku-Sopjani M. Sopjani M, et al. Curr Mol Med. 2015;15(1):27-37. doi: 10.2174/1566524015666150114111258. Curr Mol Med. 2015. PMID: 25601466 Review.
Cited by
- The anti-aging protein Klotho affects early postnatal myogenesis by downregulating Jmjd3 and the canonical Wnt pathway.
McKee CM, Chapski DJ, Wehling-Henricks M, Rosa-Garrido M, Kuro-O M, Vondriska TM, Tidball JG. McKee CM, et al. FASEB J. 2022 Mar;36(3):e22192. doi: 10.1096/fj.202101298R. FASEB J. 2022. PMID: 35174906 Free PMC article. - Aging of the immune system and impaired muscle regeneration: A failure of immunomodulation of adult myogenesis.
Tidball JG, Flores I, Welc SS, Wehling-Henricks M, Ochi E. Tidball JG, et al. Exp Gerontol. 2021 Mar;145:111200. doi: 10.1016/j.exger.2020.111200. Epub 2020 Dec 24. Exp Gerontol. 2021. PMID: 33359378 Free PMC article. Review. - Klotho as Potential Autophagy Regulator and Therapeutic Target.
Zhou H, Pu S, Zhou H, Guo Y. Zhou H, et al. Front Pharmacol. 2021 Oct 19;12:755366. doi: 10.3389/fphar.2021.755366. eCollection 2021. Front Pharmacol. 2021. PMID: 34737707 Free PMC article. Review. - Klotho: An Emerging Factor With Ergogenic Potential.
Arroyo E, Troutman AD, Moorthi RN, Avin KG, Coggan AR, Lim K. Arroyo E, et al. Front Rehabil Sci. 2022 Jan 6;2:807123. doi: 10.3389/fresc.2021.807123. eCollection 2021. Front Rehabil Sci. 2022. PMID: 36188832 Free PMC article. Review. - Relationship between klotho and physical function in healthy aging.
Arroyo E, Leber CA, Burney HN, Narayanan G, Moorthi R, Avin KG, Warden SJ, Moe SM, Lim K. Arroyo E, et al. Sci Rep. 2023 Nov 30;13(1):21158. doi: 10.1038/s41598-023-47791-5. Sci Rep. 2023. PMID: 38036596 Free PMC article.
References
- von Maltzahn J, Bentzinger CF, Rudnicki MA. Characteristics of satellite cells and multipotent adult stem cells in the skeletal muscle. Dordrecht: Springer; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources