Ion Channels and Transporters in Inflammation: Special Focus on TRP Channels and TRPC6 - PubMed (original) (raw)
Review
Ion Channels and Transporters in Inflammation: Special Focus on TRP Channels and TRPC6
Giuseppe A Ramirez et al. Cells. 2018.
Abstract
Allergy and autoimmune diseases are characterised by a multifactorial pathogenic background. Several genes involved in the control of innate and adaptive immunity have been associated with diseases and variably combine with each other as well as with environmental factors and epigenetic processes to shape the characteristics of individual manifestations. Systemic or local perturbations in salt/water balance and in ion exchanges between the intra- and extracellular spaces or among tissues play a role. In this field, usually referred to as elementary immunology, novel evidence has been recently acquired on the role of members of the transient potential receptor (TRP) channel family in several cellular mechanisms of potential significance for the pathophysiology of the immune response. TRP canonical channel 6 (TRPC6) is emerging as a functional element for the control of calcium currents in immune-committed cells and target tissues. In fact, TRPC6 influences leukocytes’ tasks such as transendothelial migration, chemotaxis, phagocytosis and cytokine release. TRPC6 also modulates the sensitivity of immune cells to apoptosis and influences tissue susceptibility to ischemia-reperfusion injury and excitotoxicity. Here, we provide a view of the interactions between ion exchanges and inflammation with a focus on the pathogenesis of immune-mediated diseases and potential future therapeutic implications.
Keywords: TRPC6; calcium; elementary immunology; endothelium; inflammation; lymphocytes; neutrophils; platelets; sodium.
Conflict of interest statement
The authors declare that they have no conflict of interest in connection with this paper.
Figures
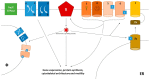
Figure 1
Ion channels and transporters. Ion channels and transporters may affect the behaviour of innate and adaptive immune cells at several levels. Under resting conditions, ion gradients between the intra- and extracellular space are actively generated through the Na/K ATPases. These gradients are exploited by transporters (1, 2) to trim the concentrations of other ions, including calcium. Cell activation after engagement of a cell-specific receptor (R), e.g., the BcR or TcR for lymphocytes or the FcR for myeloid cells, promotes the deployment of downstream signalling cascades that ultimately affect gene expression, protein synthesis and cause cytoskeletal remodelling, enabling cells to perform effector tasks such as chemotaxis, phagocytosis and release of antimicrobial moieties or cytokines. Activation of surface ion channels is integral to these events. A first set of ion channels are activated by physical or biochemical stimuli such as voltage (3), intracellular osmotic pressure (4) or engagement of extracellular (5) or intracellular (6) ligands, which in turn may be directly or indirectly induced by the activation of cell-specific receptors. Conversely, ion currents generated by voltage-operated or receptor-operated channels can exert feedback or feedforward effects on cell activating receptors. Specifically, raised calcium concentrations play a prominent role in mediating cell activation. However, to this regard store-operated calcium entry (SOCE, 7) generally provides a more significant contribution compared to voltage-operated or receptor-operated calcium entry (VOCE, ROCE). SOCE is propitiated by the activation of a inositol-1,4,5-triphosphate (IP3) receptor channel on the surface of the endoplasmic reticulum (ER, 7b). Increased intracellular IP3 concentrations are part of the changes induced by cell activation downstream cell-specific receptors (R). The release of calcium from ER stores is then sensed by adaptor proteins such as stromal interaction molecules (STIM; 7a), which in turn activate surface receptors (7), such as those of the ORAI family. Beside the cell surface, ion channels and transporters can also be expressed on intracellular compartments such as the phagolysosomes (φ). In this setting, they trim the endosomal pH, thus favouring the digestion of microbes and/or other dangerous moieties.
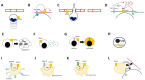
Figure 2
Effects of TRPC6 on immune cells. Activation of TRPC6 plays a critical role in the control of key cellular functions in several immune-committed cells, such as neutrophils (panel (A–D)), lymphocytes (panel (E–G)), macrophages (panel (H)), platelets (panel (I–L)) and the endothelium (panel (A–D,L)). TRPC6 contributes to neutrophil activation, adhesion to the vascular walls and extravasation by enhancing the stimulatory effects on chemo-attractants such as MIP-2 and CXCR2 (A); by promoting the downstream effects of endothelial cell adhesion molecules such as platelet/endothelial cell adhesion molecule (PECAM; (B)) or surface sensors of pro-inflammatory stimuli such as TLR-4 (D); by favouring the signal cascades that lead to looser transcellular junction between endothelial cells (C). Enhanced TRPC6 activation in lymphocytes might accelerate apoptosis, which could constitute a further trigger for inflammation in autoimmune disorders such as SLE (E). The expression of TRPC6 in T cells promotes cytokine release (F) and cell activation (G), which eventually translate in more aggressive inflammatory or allergic responses. In macrophages, TRPC6 is required for the acidification of endophagolysosomes (H). Platelets express high amounts of TRPC6 and might exploit its activation within ROCE (I,J) or SOCE (K) to undergo activation. Receptor-operated stimulation of TRPC6 downstream the thromboxane A2 (TXA2) pathway might be responsible for surface expression of crucial adhesion molecules such as GPIIb-IIIa or P-selectin (J) and for the release of platelet dense granules (J). This latter event might also occur as the result of TRPC6 activation after mobilisation of calcium from intracellular stores (K). Whether these events might impact on the interaction between platelets, leukocytes and the endothelium is still unknown (L).
Similar articles
- Intravascular adhesion and recruitment of neutrophils in response to CXCL1 depends on their TRPC6 channels.
Lindemann O, Rossaint J, Najder K, Schimmelpfennig S, Hofschröer V, Wälte M, Fels B, Oberleithner H, Zarbock A, Schwab A. Lindemann O, et al. J Mol Med (Berl). 2020 Mar;98(3):349-360. doi: 10.1007/s00109-020-01872-4. Epub 2020 Jan 16. J Mol Med (Berl). 2020. PMID: 31950205 Free PMC article. - The calcium-conducting ion channel transient receptor potential canonical 6 is involved in macrophage inflammatory protein-2-induced migration of mouse neutrophils.
Damann N, Owsianik G, Li S, Poll C, Nilius B. Damann N, et al. Acta Physiol (Oxf). 2009 Jan;195(1):3-11. doi: 10.1111/j.1748-1716.2008.01918.x. Acta Physiol (Oxf). 2009. PMID: 18983454 - Novel Targets for Stroke Therapy: Special Focus on TRPC Channels and TRPC6.
Liu L, Gu L, Chen M, Zheng Y, Xiong X, Zhu S. Liu L, et al. Front Aging Neurosci. 2020 Mar 18;12:70. doi: 10.3389/fnagi.2020.00070. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32256338 Free PMC article. Review. - TRPC6 regulates CXCR2-mediated chemotaxis of murine neutrophils.
Lindemann O, Umlauf D, Frank S, Schimmelpfennig S, Bertrand J, Pap T, Hanley PJ, Fabian A, Dietrich A, Schwab A. Lindemann O, et al. J Immunol. 2013 Jun 1;190(11):5496-505. doi: 10.4049/jimmunol.1201502. Epub 2013 May 1. J Immunol. 2013. PMID: 23636057 - TRPC6 and FSGS: the latest TRP channelopathy.
Mukerji N, Damodaran TV, Winn MP. Mukerji N, et al. Biochim Biophys Acta. 2007 Aug;1772(8):859-68. doi: 10.1016/j.bbadis.2007.03.005. Epub 2007 Mar 20. Biochim Biophys Acta. 2007. PMID: 17459670 Review.
Cited by
- Inflammation-induced TRPV4 channels exacerbate blood-brain barrier dysfunction in multiple sclerosis.
Hansen CE, Kamermans A, Mol K, Berve K, Rodriguez-Mogeda C, Fung WK, van Het Hof B, Fontijn RD, van der Pol SMA, Michalick L, Kuebler WM, Kenkhuis B, van Roon-Mom W, Liedtke W, Engelhardt B, Kooij G, Witte ME, de Vries HE. Hansen CE, et al. J Neuroinflammation. 2024 Mar 23;21(1):72. doi: 10.1186/s12974-024-03069-9. J Neuroinflammation. 2024. PMID: 38521959 Free PMC article. - M1 Macrophage Polarization Is Dependent on TRPC1-Mediated Calcium Entry.
Chauhan A, Sun Y, Sukumaran P, Quenum Zangbede FO, Jondle CN, Sharma A, Evans DL, Chauhan P, Szlabick RE, Aaland MO, Birnbaumer L, Sharma J, Singh BB, Mishra BB. Chauhan A, et al. iScience. 2018 Oct 26;8:85-102. doi: 10.1016/j.isci.2018.09.014. Epub 2018 Sep 20. iScience. 2018. PMID: 30293012 Free PMC article. - Trpc6 gain-of-function disease mutation enhances phosphatidylserine exposure in murine platelets.
Boekell KL, Brown BJ, Talbot BE, Schlondorff JS. Boekell KL, et al. PLoS One. 2022 Jun 24;17(6):e0270431. doi: 10.1371/journal.pone.0270431. eCollection 2022. PLoS One. 2022. PMID: 35749414 Free PMC article. - Identification of pathways and genes associated with meniscus degeneration using bioinformatics analyses.
Huang H, Zheng J, Deng M, Fang Y, Zhan D, Wang G. Huang H, et al. Am J Transl Res. 2021 Nov 15;13(11):12410-12420. eCollection 2021. Am J Transl Res. 2021. PMID: 34956462 Free PMC article. - Neurotrophic factor Neuritin modulates T cell electrical and metabolic state for the balance of tolerance and immunity.
Yu H, Nishio H, Barbi J, Mitchell-Flack M, Vignali PDA, Zheng Y, Lebid A, Chang KY, Fu J, Higgins M, Huang CT, Zhang X, Li Z, Blosser L, Tam A, Drake CG, Pardoll DM. Yu H, et al. bioRxiv [Preprint]. 2024 Aug 7:2024.01.31.578284. doi: 10.1101/2024.01.31.578284. bioRxiv. 2024. PMID: 38352414 Free PMC article. Updated. Preprint.
References
- European Bioinformatics Institute (EMBL-EBI) SIB Swiss Institute of Bioinformatics. (PIR), P.I.R Universal Protein Resource (Uniprot) [(accessed on 5 June 2018)]; Available online: http://www.uniprot.org/
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources