Different infusion durations for preventing platinum-induced hearing loss in children with cancer - PubMed (original) (raw)
Review
Different infusion durations for preventing platinum-induced hearing loss in children with cancer
Jorrit W van As et al. Cochrane Database Syst Rev. 2018.
Update in
- Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2020 Jan 21;1(1):CD010885. doi: 10.1002/14651858.CD010885.pub5. Cochrane Database Syst Rev. 2020. PMID: 31961948 Free PMC article.
Abstract
Background: Platinum-based therapy, including cisplatin, carboplatin or oxaliplatin, or a combination of these, is used to treat a variety of paediatric malignancies. Unfortunately, one of the most important adverse effects is the occurrence of hearing loss or ototoxicity. In an effort to prevent this ototoxicity, different platinum infusion durations have been studied. This review is the second update of a previously published Cochrane review.
Objectives: To assess the effects of different durations of platinum infusion to prevent hearing loss or tinnitus, or both, in children with cancer. Secondary objectives were to assess possible effects of these infusion durations on: a) anti-tumour efficacy of platinum-based therapy, b) adverse effects other than hearing loss or tinnitus, and c) quality of life.
Search methods: We searched the electronic databases Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library 15 March 2018), MEDLINE (PubMed) (1945 to 15 March 2018) and Embase (Ovid) (1980 to 15 March 2018). In addition, we handsearched reference lists of relevant articles and we assessed the conference proceedings of the International Society for Paediatric Oncology (2009 up to and including 2017) and the American Society of Pediatric Hematology/Oncology (2014 up to and including 2017). We scanned ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch) for ongoing trials (searched on 12 March 2018 and 13 March 2018 respectively).
Selection criteria: Randomised controlled trials (RCTs) or controlled clinical trials (CCTs) comparing different platinum infusion durations in children with cancer. Only the platinum infusion duration could differ between the treatment groups.
Data collection and analysis: Two review authors independently performed the study selection, 'Risk of bias' assessment and GRADE assessment of included studies, and data extraction including adverse effects. Analyses were performed according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results: We identified one RCT and no CCTs; in this update no additional studies were identified. The RCT (total number of children = 91) evaluated the use of a continuous cisplatin infusion (N = 43) versus a one-hour bolus cisplatin infusion (N = 48) in children with neuroblastoma. For the continuous infusion, cisplatin was administered on days one to five of the cycle, but it is unclear if the infusion duration was a total of five days. Risk of bias was present. Only results from shortly after induction therapy were provided. No clear evidence of a difference in hearing loss (defined as asymptomatic and symptomatic disease combined) between the different infusion durations was identified as results were imprecise (risk ratio (RR) 1.39, 95% confidence interval (CI) 0.47 to 4.13, low-quality evidence). Although the numbers of children were not provided, it was stated that tumour response was equivalent in both treatment arms. With regard to adverse effects other than ototoxicity, we were only able to assess toxic deaths. Again, the confidence interval of the estimated effect was too wide to exclude differences between the treatment groups (RR 1.12, 95% CI 0.07 to 17.31, low-quality evidence). No data were available for the other outcomes of interest (i.e. tinnitus, overall survival, event-free survival and quality of life) or for other (combinations of) infusion durations or other platinum analogues.
Authors' conclusions: Since only one eligible RCT evaluating the use of a continuous cisplatin infusion versus a one-hour bolus cisplatin infusion was found, and that had methodological limitations, no definitive conclusions can be made. It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. For other (combinations of) infusion durations and other platinum analogues no eligible studies were identified. More high-quality research is needed.
Conflict of interest statement
None known.
Figures
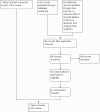
Figure 1
Flow diagram of selection of studies
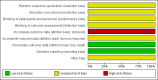
Figure 2
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3
Forest plot of comparison: 1 Continuous platinum infusion versus bolus platinum infusion, outcome: 1.1 Hearing loss (asymptomatic and symptomatic disease).
Figure 4
Forest plot of comparison: 1 Continuous platinum infusion versus bolus platinum infusion, outcome: 1.2 Adverse effects: toxic death.
Analysis 1.1
Comparison 1 Continuous platinum infusion versus bolus platinum infusion, Outcome 1 Hearing loss (asymptomatic and symptomatic disease).
Analysis 1.2
Comparison 1 Continuous platinum infusion versus bolus platinum infusion, Outcome 2 Adverse effects: toxic death.
Update of
- Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2016 Aug 8;(8):CD010885. doi: 10.1002/14651858.CD010885.pub3. Cochrane Database Syst Rev. 2016. PMID: 27498707 Updated. Review.
Similar articles
- Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2020 Jan 21;1(1):CD010885. doi: 10.1002/14651858.CD010885.pub5. Cochrane Database Syst Rev. 2020. PMID: 31961948 Free PMC article. - Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2016 Aug 8;(8):CD010885. doi: 10.1002/14651858.CD010885.pub3. Cochrane Database Syst Rev. 2016. PMID: 27498707 Updated. Review. - Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2014 Jun 26;(6):CD010885. doi: 10.1002/14651858.CD010885.pub2. Cochrane Database Syst Rev. 2014. PMID: 24968257 Updated. Review. - Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2019 May 7;5(5):CD009219. doi: 10.1002/14651858.CD009219.pub5. Cochrane Database Syst Rev. 2019. PMID: 31063591 Free PMC article. - Medical interventions for the prevention of platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2014 Jul 1;(7):CD009219. doi: 10.1002/14651858.CD009219.pub3. Cochrane Database Syst Rev. 2014. PMID: 24984156 Review.
Cited by
- Upfront Treatment of Pediatric High-Risk Neuroblastoma With Chemotherapy, Surgery, and Radiotherapy Combination: The CCCG-NB-2014 Protocol.
Zhang D, Kaweme NM, Duan P, Dong Y, Yuan X. Zhang D, et al. Front Oncol. 2021 Nov 15;11:745794. doi: 10.3389/fonc.2021.745794. eCollection 2021. Front Oncol. 2021. PMID: 34868944 Free PMC article. - Long-term follow-up of high-risk neuroblastoma survivors treated with high-dose chemotherapy and stem cell transplantation rescue.
Haghiri S, Fayech C, Mansouri I, Dufour C, Pasqualini C, Bolle S, Rivollet S, Dumas A, Boumaraf A, Belhout A, Journy N, Souchard V, Vu-Bezin G, Veres C, Haddy N, De Vathaire F, Valteau-Couanet D, Fresneau B. Haghiri S, et al. Bone Marrow Transplant. 2021 Aug;56(8):1984-1997. doi: 10.1038/s41409-021-01258-1. Epub 2021 Apr 6. Bone Marrow Transplant. 2021. PMID: 33824435 - Pharmacotherapy of Tinnitus.
Kleinjung T, Langguth B. Kleinjung T, et al. Curr Top Behav Neurosci. 2021;51:193-212. doi: 10.1007/7854_2020_169. Curr Top Behav Neurosci. 2021. PMID: 32761509 Review. - Ursolic acid protects against cisplatin‑induced ototoxicity by inhibiting oxidative stress and TRPV1‑mediated Ca2+‑signaling.
Di Y, Xu T, Tian Y, Ma T, Qu D, Wang Y, Lin Y, Bao D, Yu L, Liu S, Wang A. Di Y, et al. Int J Mol Med. 2020 Aug;46(2):806-816. doi: 10.3892/ijmm.2020.4633. Epub 2020 Jun 4. Int J Mol Med. 2020. PMID: 32626955 Free PMC article. - Different infusion durations for preventing platinum-induced hearing loss in children with cancer.
van As JW, van den Berg H, van Dalen EC. van As JW, et al. Cochrane Database Syst Rev. 2020 Jan 21;1(1):CD010885. doi: 10.1002/14651858.CD010885.pub5. Cochrane Database Syst Rev. 2020. PMID: 31961948 Free PMC article.
References
References to studies included in this review
- Coze C, Hartmann O, Michon J, Frappaz D, Dusol F, Rubie H, et al. NB87 induction protocol for stage 4 neuroblastoma in children over 1 year of age: a report from the French Society of Pediatric Oncology. Journal of Clinical Oncology 1997;15(12):3433‐40. - PubMed
References to studies excluded from this review
- Bergeron C, Dubourg L, Chastagner P, Mechinaud F, Plouvier E, Desfachelles AS, et al. Long‐term renal and hearing toxicity of carboplatin in infants treated for localized and unresectable neuroblastoma: results of the SFOP NBL90 study. Pediatric Blood & Cancer 2005;45(1):32‐6. - PubMed
- Lanvers‐Kaminsky C, Krefeld B, Dinnesen AG, Deuster D, Seifert E, Würthwein G, et al. Continuous or repeated prolonged cisplatin infusions in children: a prospective study on ototoxicity, platinum concentrations, and standard serum parameters. Pediatric Blood & Cancer 2006;47(2):183‐93. - PubMed
Additional references
- Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, et al. Platinum compound‐related ototoxicity in children: long‐term follow‐up reveals continuous worsening of hearing loss. Journal of Pediatric Hematology/Oncology 2004;26(10):649‐55. - PubMed
- Brock P, Yoeman L, Bellman S, Pritchard J. Ototoxicity in children treated with cis‐platinum (CDDP) for germ cell (GCT) and other tumours. Medical and Pediatric Oncology. 1987; Vol. 15:329 (abstract 138).
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Medical and Pediatric Oncology 1991;19(4):295‐300. - PubMed
- Dean JB, Hayashi SS, Albert CM, King AA, Karzon R, Hayashi RJ. Hearing loss in pediatric oncology patients receiving carboplatin‐containing regimens. Journal of Pediatric Hematology/Oncology 2008;30(2):130‐4. - PubMed
- Eloxatin Summary of Product Characteristics. www.sanofi‐aventis.co.uk/products/Eloxatin_SPC.pdf (Accessed 2 March 2010).
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical