Routine determination of ice thickness for cryo-EM grids - PubMed (original) (raw)
Routine determination of ice thickness for cryo-EM grids
William J Rice et al. J Struct Biol. 2018 Oct.
Abstract
Recent advances in instrumentation and automation have made cryo-EM a popular method for producing near-atomic resolution structures of a variety of proteins and complexes. Sample preparation is still a limiting factor in collecting high quality data. Thickness of the vitreous ice in which the particles are embedded is one of the many variables that need to be optimized for collection of the highest quality data. Here we present two methods, using either an energy filter or scattering outside the objective aperture, to measure ice thickness for potentially every image collected. Unlike geometrical or tomographic methods, these can be implemented directly in the single particle collection workflow without interrupting or significantly slowing down data collection. We describe the methods as implemented into the Leginon/Appion data collection workflow, along with some examples from test cases. Routine monitoring of ice thickness should prove helpful for optimizing sample preparation, data collection, and data processing.
Keywords: Cryo-EM; Energy filter; Ice thickness; Inelastic scattering; Mean free path.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
Figure 1.
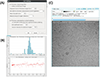
(A) Representative grid atlas collected in Leginon, showing an evident gradient in ice thickness. (B) A “square” level image from this same grid shows evidence of varying thickness, including occasional empty holes.
Figure 2.
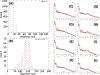
(A) Determination of mean free path for inelastic scattering by electron tomography. Images were collected for both aldolase and proteasome samples with and without a 15 eV energy slit. Tomograms were collected at the same areas as thickness was measured. Thickness versus Log (Itot/IZLP) is plotted. The line of best fit (green) had a slope of 395 nm. (B) Thickness determined using the energy filter for untilted and 45 degree tilted images. Inset: an overview of 4 holes are shown where the measurements were made, untilted (left) and tilted (right). For each hole, thickness was measured as described for both tilted and untilted images, and plotted as tilted thickness versus untilted thickness (purple crosses). The green line is a plot of the equation y=1.41x, which is where the points should ideally lie. (C) Determination of the ALS coefficient using thickness as measured by the energy filter. Thickness was measured over many images using the energy filter and eq. (1). Thickness plotted versus log I0/I as described in the text. For this experiment, the slope was 332 nm.
Figure 3.
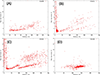
(A) Histogram of thickness values as measured on a rabbit muscle aldolase sample frozen on gold Ultrafoil grids. (B) Histogram of thickness values as measured on a T20S proteasome test sample frozen on C-flat carbon grids. (C-J) Plots of mean radial intensity of Fourier transforms of image averages versus resolution for various ice thicknesses. Blue bar: 3.9 Å−1 resolution. (C): 0–25 nm thickness. (D): 25–50 nm thickness. (E): 50–75 nm thickness. (F) 75–100 nm thickness. (G): 100–125 nm thickness. (H): 125–150 nm thickness. (I): 150–175 nm thickness. (J): 175–200 nm thickness.
Figure 4:
Plots of Thon ring extent, as measured by CTFFIND4 (Å−1), versus ice thickness for several samples. (A): glutamate dehydrogenase. (B, C): rabbit muscle aldolase. (D): T20S proteasome. Thon ring extent goes to very low resolution where the ice is too thin or absent, is optimal at thin ice, and generally rises as ice thickens.
Figure 5:
Integration of ice thickness determination into Leginon. (A) The control panel for the node is shown. The user provides various parameters for thickness determination and chooses how often the thickness is measured. (B) Thickness plots for an experiment as shown in the Appion summary pages. (C) Thickness information is displayed when viewing the image in the Leginon web interface.
Similar articles
- Optimized cryo-EM data-acquisition workflow by sample-thickness determination.
Rheinberger J, Oostergetel G, Resch GP, Paulino C. Rheinberger J, et al. Acta Crystallogr D Struct Biol. 2021 May 1;77(Pt 5):565-571. doi: 10.1107/S205979832100334X. Epub 2021 Apr 27. Acta Crystallogr D Struct Biol. 2021. PMID: 33950013 Free PMC article. - Ice thickness monitoring for cryo-EM grids by interferometry imaging.
Hohle MM, Lammens K, Gut F, Wang B, Kahler S, Kugler K, Till M, Beckmann R, Hopfner KP, Jung C. Hohle MM, et al. Sci Rep. 2022 Sep 12;12(1):15330. doi: 10.1038/s41598-022-16978-7. Sci Rep. 2022. PMID: 36097274 Free PMC article. - Routine single particle CryoEM sample and grid characterization by tomography.
Noble AJ, Dandey VP, Wei H, Brasch J, Chase J, Acharya P, Tan YZ, Zhang Z, Kim LY, Scapin G, Rapp M, Eng ET, Rice WJ, Cheng A, Negro CJ, Shapiro L, Kwong PD, Jeruzalmi D, des Georges A, Potter CS, Carragher B. Noble AJ, et al. Elife. 2018 May 29;7:e34257. doi: 10.7554/eLife.34257. Elife. 2018. PMID: 29809143 Free PMC article. - Routine Collection of High-Resolution cryo-EM Datasets Using 200 KV Transmission Electron Microscope.
Koh A, Khavnekar S, Yang W, Karia D, Cats D, van der Ploeg R, Grollios F, Raschdorf O, Kotecha A, Němeček D. Koh A, et al. J Vis Exp. 2022 Mar 16;(181). doi: 10.3791/63519. J Vis Exp. 2022. PMID: 35377368 Review. - Approaches to altering particle distributions in cryo-electron microscopy sample preparation.
Drulyte I, Johnson RM, Hesketh EL, Hurdiss DL, Scarff CA, Porav SA, Ranson NA, Muench SP, Thompson RF. Drulyte I, et al. Acta Crystallogr D Struct Biol. 2018 Jun 1;74(Pt 6):560-571. doi: 10.1107/S2059798318006496. Epub 2018 May 18. Acta Crystallogr D Struct Biol. 2018. PMID: 29872006 Free PMC article. Review.
Cited by
- Understanding the invisible hands of sample preparation for cryo-EM.
Weissenberger G, Henderikx RJM, Peters PJ. Weissenberger G, et al. Nat Methods. 2021 May;18(5):463-471. doi: 10.1038/s41592-021-01130-6. Epub 2021 May 7. Nat Methods. 2021. PMID: 33963356 Review. - Structure and drug resistance of the Plasmodium falciparum transporter PfCRT.
Kim J, Tan YZ, Wicht KJ, Erramilli SK, Dhingra SK, Okombo J, Vendome J, Hagenah LM, Giacometti SI, Warren AL, Nosol K, Roepe PD, Potter CS, Carragher B, Kossiakoff AA, Quick M, Fidock DA, Mancia F. Kim J, et al. Nature. 2019 Dec;576(7786):315-320. doi: 10.1038/s41586-019-1795-x. Epub 2019 Nov 27. Nature. 2019. PMID: 31776516 Free PMC article. - Thinner is not always better: Optimizing cryo-lamellae for subtomogram averaging.
Tuijtel MW, Cruz-León S, Kreysing JP, Welsch S, Hummer G, Beck M, Turoňová B. Tuijtel MW, et al. Sci Adv. 2024 Apr 26;10(17):eadk6285. doi: 10.1126/sciadv.adk6285. Epub 2024 Apr 26. Sci Adv. 2024. PMID: 38669330 Free PMC article. - Assessing the JEOL CRYO ARM 300 for high-throughput automated single-particle cryo-EM in a multiuser environment.
Fislage M, Shkumatov AV, Stroobants A, Efremov RG. Fislage M, et al. IUCrJ. 2020 Jun 11;7(Pt 4):707-718. doi: 10.1107/S2052252520006065. eCollection 2020 Jul 1. IUCrJ. 2020. PMID: 32695417 Free PMC article. - Batch Production of High-Quality Graphene Grids for Cryo-EM: Cryo-EM Structure of Methylococcus capsulatus Soluble Methane Monooxygenase Hydroxylase.
Ahn E, Kim B, Park S, Erwin AL, Sung SH, Hovden R, Mosalaganti S, Cho US. Ahn E, et al. ACS Nano. 2023 Mar 28;17(6):6011-6022. doi: 10.1021/acsnano.3c00463. Epub 2023 Mar 16. ACS Nano. 2023. PMID: 36926824 Free PMC article.
References
- Adrian M, Dubochet J, Lepault J, McDowall AW, 1984. Cryo-electron microscopy of viruses. Nature 308, 32–36. - PubMed
- Angert I, Burmester C, Dinges C, Rose H, Schroder RR, 1996. Elastic and inelastic scattering cross-sections of amorphous layers of carbon and vitrified ice. Ultramicroscopy 63, 181–192.
- Bierhoff MPMD,NL), Kooijman, Sander Kees Cornelis (Veldhoven NL), Sanford, Colin August (Atkinson, NH, US). 2005. Magnetic lens.
- Biyani N, Righetto RD, McLeod R, Caujolle-Bert D, Castano-Diez D, Goldie KN, Stahlberg H, 2017. Focus: The interface between data collection and data processing in cryo-EM. J Struct Biol 198, 124–133. - PubMed
- Brydson R, Royal Microscopical Society (Great Britain), 2001. Electron energy loss spectroscopy Bios in association with the Royal Microscopical Society, Oxford.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources