A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells - PubMed (original) (raw)
A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells
Francesca Diomede et al. Int J Nanomedicine. 2018.
Abstract
Purpose: The combination of oral derived stem cells and 3-D scaffolds is considered advantageous in bone repair. In particular, collagen membranes possess ideal biological properties and can support infiltration and proliferation of osteoblasts, promoting bone regeneration. Our study aimed to develop a new biocompatible osteogenic construct composed of a commercially available collagen membrane (Evolution [Evo]), human periodontal-ligament stem cells (hPDLSCs) enriched with extracellular vesicles (EVs), or polyethylenimine (PEI)-engineered EVs (PEI-EVs).
Methods: Osteogenic ability and expression of osteogenic genes were evaluated in vitro in hPDLSCs cultured with or without Evo, with Evo and EVs, or PEI-EVs. In addition, the bone-regeneration capacity of Evo, Evo enriched with hPDLSCs, Evo enriched with hPDLSCs and EVs/PEI-EVs was investigated in rats subjected to calvarial defects.
Results: Our results showed that Evo enriched with EVs and PEI-EVs showed high biocompatibility and osteogenic properties in vitro and in vivo. In addition, quantitative reverse-transcription polymerase chain reaction demonstrated the upregulation of osteogenic genes, such as TGFB1, MMP8, TUFT1, TFIP11, BMP2, and BMP4, in the presence of PEI-EVs. Upregulation of BMP2/4 was confirmed for Evo enriched with PEI-EVs and hPDLSCs both in vitro by Western blot and in vivo by immunofluorescence.
Conclusion: Our results indicated that Evo enriched with hPDLSCs and PEI-EVs is able to promote a bone-regeneration process for the treatment of calvarium and ossification defects caused by accidental or surgery trauma. In particular, PEI-EVs had a significant role in activation of the osteogenic process.
Keywords: bone regeneration; collagen membrane; extracellular vesicles; human periodontal-ligament stem cells; living construct.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Figure 1
Topographical analysis of EVs and PEI-EV and features of PEI-EVs with varying PEI concentrations. Notes: (A) EVs increased in dimension after the addition of PEI, and in particular PEI-EVs obtained using higher concentrations of PEI were larger than those obtained with lower PEI doses, with the exception of the 0.05 mg/mL PEI sample. (B) ζ-potential increased with higher PEI concentrations. The ζ-potential of −1.2±0.9 mV may explain the higher dimensions for PEI-EVs obtained with 0.05 mg/mL PEI, because the decreased electrostatic repulsions promoted PEI-EV aggregation. (C) EVs analyzed by tapping-mode topographic 3-D AFM were globular with a central depression. (D) PEI-EVs obtained using PEI 0.05 mg/mL, analyzed by tapping-mode topographic 3-D AFM, showed globular morphology without the central depression and less smooth surfaces. Abbreviations: EVs, extracellular vesicles; PEI, polyethylenimine; AFM, atomic force microscopy.
Figure 2
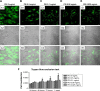
Confocal laser-scanning microscopy of PEI-EV (stained green with WGA Alexa Fluor 488) and hPDLSC (gray) interaction using different PEI concentrations. Notes: hPDLSCs incubated with EVs coated with PEI at 5 mg/mL (A1–A3); 0.5 mg/mL (B1–B3); 0.1 mg/mL (C1–C3), 0.05 mg/mL (D1–D3), and 0.025 mg/mL (E1–E3). PEI-EVs stained in green with WGA Alexa Fluor 488 (A1–E1). Light-transmission channels showed cell morphology (gray; A2–E2); merged images of the aforementioned channels (A3–E3). (F) Trypan blue exclusion test to evaluate the effects of different PEI concentrations on hPDLSC viability. 0.05 mg/mL PEI concentration was chosen for the other experiments. **P<0.01 compared to the other PEI concentrations; n=3; scale bars =10 μm. Abbreviations: PEI, polyethylenimine; EV, extracellular vesicle; WGA, wheat-germ agglutinin; hPDLSC, human periodontal-ligament stem cell.
Figure 3
Confocal laser-scanning microscopy showing Evo coated with EVs and PEI-EVs. Notes: (A) Stained EVs (green) seeded on the Evo membrane. (D) Stained-PEI-EVs (green) seeded on the Evo membrane. Microphotography confirmed the presence of EVs on the Evo surface. (B and E) The light-transmission channel shows Evo morphology (gray). (C and F) Merged images of the aforementioned channels. Microphotography confirmed the presence of PEI-EVs on the Evo surface. PEI-EVs and EVs showed the same capacity to cover the Evo surface. Scale bars =10 μm. Abbreviations: EVs, extracellular vesicles; PEI, polyethylenimine; Evo, Evolution.
Figure 4
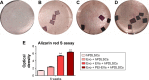
Evo, hPDLSCs, EVs, and PEI-EVs and osteogenic differentiation. Notes: Photography after 6 weeks of culture and stained with Alizarin red S solution of (A) hPDLSCs, (B) Evo + hPDLSCs, (C) Evo + EVs + hPDLSCs, and (D) Evo + PEI-EVs + hPDLSCs showed that the best results in terms of production of calcium deposition were visible in Evo + PEI-EVs + hPDLSCs. (E) Colorimetric detection quantification. **P<0.01 compared to hPDLSCs. Abbreviations: Evo, Evolution; hPDLSCs, human periodontal-ligament stem cells; EVs, extracellular vesicles; PEI, polyethylenimine.
Figure 5
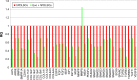
Relative gene-expression fold changes by qRT-PCR in Evo + hPDLSCs. Notes: Evo + hPDLSCs compared to hPDLSCs. Evo + hPDLSCs induced the modulation of 40 genes, with only MMP8 upregulated. Transcripts showed a _P_-value <0.05; _P_-values adjusted using Benjamini–Hochberg false-discovery-rate correction. Abbreviations: qRT-PCR, quantitative reverse-transcription polymerase chain reaction; Evo, Evolution; hPDLSCs, human periodontal-ligament stem cells; RQ, relative quantification.
Figure 6
Relative gene-expression fold changes by qRT-PCR in Evo + EVs + hPDLSCs. Notes: Evo + EVs + hPDLSCs compared to hPDLSCs. Cells cultured with Evo + EVs showed modulation of 36 genes, with only TGFB1 and TGFB2 upregulated. Transcripts show a _P_-value <0.05; _P_-values adjusted using Benjamini–Hochberg false-discovery-rate correction. Abbreviations: qRT-PCR, quantitative reverse-transcription polymerase chain reaction; Evo, Evolution; EVs, extracellular vesicles; hPDLSCs, human periodontal-ligament stem cells; RQ, relative quantification.
Figure 7
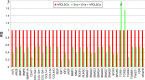
Relative gene-expression fold changes by qRT-PCR in Evo + PEI-EVs + hPDLSCs. Notes: Evo + PEI-EVs + hPDLSCs compared to hPDLSCs. Cells cultured with Evo + PEI-EVs showed modulation of 40 transcripts with upregulation of nine genes, including BMP2, BMP4, COL, and TGFB1. Transcripts show a _P_-value <0.05; _P_-values adjusted using Benjamini–Hochberg false-discovery-rate correction. Abbreviations: qRT-PCR, quantitative reverse-transcription polymerase chain reaction; Evo, Evolution; PEI, polyethylenimine; EVs, extracellular vesicles; hPDLSCs, human periodontal-ligament stem cells; RQ, relative quantification.
Figure 8
Macroscopic images of rat calvaria. Notes: Six weeks after the implant of Evo, Evo + hPDLSCs, Evo + PEI-EVs, and Evo + PEI-EVs + hPDLSCs. Macroscopic evaluation showed almost complete bone repair in the Evo + PEI-EVs + hPDLSC group, while in the other groups the lesion was still present. Rectangles enclose the damaged area. Abbreviations: Evo, Evolution; hPDLSCs, human periodontal-ligament stem cells; PEI, polyethylenimine; EVs, extracellular vesicles.
Figure 9
Histological evaluation after 6 weeks of healing. Notes: Magnification is 4× for A1–F1 and 40× for A2–F2. (A) Evo. Membrane appeared structurally intact, able to cover all the defect area, and well integrated with the host tissue. (B) Evo + hPDLSCs. This group showed good integration with vascularized extracellular matrix around the native bone. (C) Evo + EVs. Islets of new bone were integrated with native bone. Membrane appeared closely adapted to host-bone tissue and well integrated with native bone. (D) Evo + EVs + hPDLSCs. (E) Evo + PEI-EVs. Evo was well integrated with host tissue, and extracellular matrix was present in defect area. (F) Evo + PEI-EVs + hPDLSCs. The implant site was covered with an organized extracellular matrix with mineralization areas, and osteoblast-like structures were visible. C, mouse calvarium; *Evo; arrows, osteoblast-like cells. Scale bars =10 μm. Abbreviations: Evo, Evolution; hPDLSCs, human periodontal-ligament stem cells; EVs, extracellular vesicles; PEI, polyethylenimine.
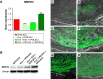
Figure 10
In vitro and in vivo BMP2/4 expression. Notes: (A) qRT-PCR graph showing different BMP2/4 expression in hPDLSCs, Evo + hPDLSCs, Evo + EVs + hPDLSCs, and Evo + PEI-EVs + hPDLSCs after 6 weeks of in vitro culture (n=3, **P<0.01 compared to hPDLSCs, Evo + hPDLSCs, and Evo + EVs + hPDLSCs). Evo + PEI-EVs + hPDLSCs showed the highest expression. (B) Western blot analysis of BMP2/4 confirmed the gene-expression results. Immunofluorescence staining of BMP2/4 showed the presence of the protein in semithin section samples obtained after 6 weeks of grafting in rat calvaria in (C) Evo, (D) Evo + hPDLSCs, (E) Evo + EVs, (F) Evo + EVs + hPDLSCs, (G) Evo + PEI-EVs, and (H) Evo + PEI-EVs + hPDLSCs. The results showed higher protein expression in Evo + PEI-EVs + hPDLSCs, confirming the in vitro data. Magnification 20×; C, mouse calvarium; *Evo; Scale bars =10 μm. Abbreviations: qRT-PCR, quantitative reverse-transcription polymerase chain reaction; hPDLSCs, human periodontal-ligament stem cells; Evo, Evolution; EVs, extracellular vesicles; PEI, polyethylenimine.
Similar articles
- Engineered Extracellular Vesicles From Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration.
Pizzicannella J, Gugliandolo A, Orsini T, Fontana A, Ventrella A, Mazzon E, Bramanti P, Diomede F, Trubiani O. Pizzicannella J, et al. Front Physiol. 2019 Apr 30;10:512. doi: 10.3389/fphys.2019.00512. eCollection 2019. Front Physiol. 2019. PMID: 31114512 Free PMC article. - PWAR6 interacts with miR‑106a‑5p to regulate the osteogenic differentiation of human periodontal ligament stem cells.
Xiang J, Bian Y. Xiang J, et al. Mol Med Rep. 2021 Apr;23(4):268. doi: 10.3892/mmr.2021.11907. Epub 2021 Feb 12. Mol Med Rep. 2021. PMID: 33576453 Free PMC article. - Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair.
Trubiani O, Marconi GD, Pierdomenico SD, Piattelli A, Diomede F, Pizzicannella J. Trubiani O, et al. Int J Mol Sci. 2019 Oct 9;20(20):4987. doi: 10.3390/ijms20204987. Int J Mol Sci. 2019. PMID: 31600975 Free PMC article. Review. - Advances in the Study of Extracellular Vesicles for Bone Regeneration.
Jiao Y, Liu Y, Du J, Xu J, Luo Z, Liu Y, Guo L. Jiao Y, et al. Int J Mol Sci. 2024 Mar 20;25(6):3480. doi: 10.3390/ijms25063480. Int J Mol Sci. 2024. PMID: 38542453 Free PMC article. Review.
Cited by
- 3D Human Periodontal Stem Cells and Endothelial Cells Promote Bone Development in Bovine Pericardium-Based Tissue Biomaterial.
Pizzicannella J, Pierdomenico SD, Piattelli A, Varvara G, Fonticoli L, Trubiani O, Diomede F. Pizzicannella J, et al. Materials (Basel). 2019 Jul 5;12(13):2157. doi: 10.3390/ma12132157. Materials (Basel). 2019. PMID: 31284396 Free PMC article. - Extracellular vesicles in bone and periodontal regeneration: current and potential therapeutic applications.
Gholami L, Nooshabadi VT, Shahabi S, Jazayeri M, Tarzemany R, Afsartala Z, Khorsandi K. Gholami L, et al. Cell Biosci. 2021 Jan 12;11(1):16. doi: 10.1186/s13578-020-00527-8. Cell Biosci. 2021. PMID: 33436061 Free PMC article. Review. - Stem Cells Secretome from Oral Tissue Could Represent a Promising Therapeutic Approach in COVID-19-Disease?
Diomede F, Marconi GD, Fonticoli L, Pizzicannella J, Trubiani O. Diomede F, et al. Int J Mol Sci. 2020 Sep 17;21(18):6833. doi: 10.3390/ijms21186833. Int J Mol Sci. 2020. PMID: 32957696 Free PMC article. - Dental stem cell-derived extracellular vesicles as promising therapeutic agents in the treatment of diseases.
Li Y, Duan X, Chen Y, Liu B, Chen G. Li Y, et al. Int J Oral Sci. 2022 Jan 4;14(1):2. doi: 10.1038/s41368-021-00152-2. Int J Oral Sci. 2022. PMID: 34980877 Free PMC article. Review. - Hydrogels: 3D Drug Delivery Systems for Nanoparticles and Extracellular Vesicles.
Chabria Y, Duffy GP, Lowery AJ, Dwyer RM. Chabria Y, et al. Biomedicines. 2021 Nov 15;9(11):1694. doi: 10.3390/biomedicines9111694. Biomedicines. 2021. PMID: 34829923 Free PMC article. Review.
References
- Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81(5):672–676. - PubMed
- Basha RY, Kumar TS, Doble M. Design of biocomposite materials for bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2015;57:452–463. - PubMed
- Ferreira AM, Gentile P, Chiono V, Ciardelli G. Collagen for bone tissue regeneration. Acta Biomater. 2012;8(9):3191–3200. - PubMed
- Chu C, Deng J, Hou Y, et al. Application of PEG and EGCG modified collagen-base membrane to promote osteoblasts proliferation. Mater Sci Eng C Mater Biol Appl. 2017;76:31–36. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous