Synaptic Vesicle Endocytosis in Different Model Systems - PubMed (original) (raw)
Review
Synaptic Vesicle Endocytosis in Different Model Systems
Quan Gan et al. Front Cell Neurosci. 2018.
Abstract
Neurotransmission in complex animals depends on a choir of functionally distinct synapses releasing neurotransmitters in a highly coordinated manner. During synaptic signaling, vesicles fuse with the plasma membrane to release their contents. The rate of vesicle fusion is high and can exceed the rate at which synaptic vesicles can be re-supplied by distant sources. Thus, local compensatory endocytosis is needed to replenish the synaptic vesicle pools. Over the last four decades, various experimental methods and model systems have been used to study the cellular and molecular mechanisms underlying synaptic vesicle cycle. Clathrin-mediated endocytosis is thought to be the predominant mechanism for synaptic vesicle recycling. However, recent studies suggest significant contribution from other modes of endocytosis, including fast compensatory endocytosis, activity-dependent bulk endocytosis, ultrafast endocytosis, as well as kiss-and-run. Currently, it is not clear whether a universal model of vesicle recycling exist for all types of synapses. It is possible that each synapse type employs a particular mode of endocytosis. Alternatively, multiple modes of endocytosis operate at the same synapse, and the synapse toggles between different modes depending on its activity level. Here we compile review and research articles based on well-characterized model systems: frog neuromuscular junctions, C. elegans neuromuscular junctions, Drosophila neuromuscular junctions, lamprey reticulospinal giant axons, goldfish retinal ribbon synapses, the calyx of Held, and rodent hippocampal synapses. We will compare these systems in terms of their known modes and kinetics of synaptic vesicle endocytosis, as well as the underlying molecular machineries. We will also provide the future development of this field.
Keywords: kinetics of endocytosis; model systems; molecular mechanisms; synaptic vesicle endocytosis; synaptic vesicle recycling.
Figures
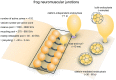
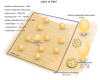
Figure 1
Vesicle pools and endocytic pathways at frog neuromuscular junctions. Frog neuromuscular junctions possess on average 110 active zones. At each active zone, 30–40 vesicles are docked on either side of a central “ridge.” These vesicles represent the readily-releasable pool (RRP). The number of recycling pool vesicles per active zone is 270–680, and the number of reserve pool vesicles per active zone is 1500–3800. During physiological stimulation, vesicle recycling takes place on a time scale of 30 s−2 min. Two endocytic pathways are found at frog neuromuscular junctions: a fast pathway that internalizes vesicles and cisternae within a minute, and a slow pathway that internalizes vesicles at ~8 min during intense stimulation by high K+. The fast pathway is mediated by clathrin-independent endocytosis (potentially ultrafast endocytosis or fast compensatory endocytosis) and clathrin-mediated endocytosis. The slow pathway represents activity-dependent bulk endocytosis. An alternative fast pathway kiss-and-run is also suggested. This pathway is predicted to occur within 10 ms. However, the existence of kiss-and-run at frog neuromuscular junctions is still controversial.
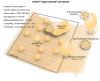
Figure 2
Vesicle pools and endocytic pathways at C. elegans neuromuscular junctions. C. elegans neuromuscular junctions usually possess a single active zone with a dense projection. An average of 35 vesicles are docked, of which 10–15 can fuse in response to a single stimulus, and therefore represents the RRP. The rest of the docked vesicles (15–20) represent the recycling pool. Roughly 240 vesicles are in the reserve pool. Following single stimuli, ultrafast endocytosis occurs on a time scale of 20 ms–1 s both at the dense projection or at adherens junctions. Bulk endocytosis might also occur at this synapse following hyperstimulation (not shown in figure).
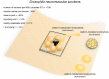
Figure 3
Vesicle pools and endocytic pathways at Drosophila neuromuscular junctions. Drosophila neuromuscular junctions possess 200–300 active zones. The center of each active zone is marked by a dense projection commonly referred to as a “T-bar”, around which synaptic vesicles tend to cluster. In each bouton, 0.7% of the vesicles on average are readily releasable; ~14% of the vesicles are in the recycling pool; and roughly ~85% are in the reserve pool. Synaptic vesicle endocytosis occurs with a time constant of 14 s as measured by pHluorin. Both clathrin-mediated and clathrin-independent endocytosis occurs at Drosophila neuromuscular junctions. One particular form of clathrin-independent endocytosis might occur adjacent to the T-bar. The exact time courses of these endocytic pathways have not been determined.
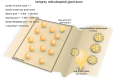
Figure 4
Vesicle pools and endocytic pathways at lamprey reticulospinal giant synapses. Lamprey reticulospinal giant synapses usually possess only one active zone. The exact number of vesicles in the RRP is unknown. Vesicles at this synapse can be divided into a synapsin-independent (recycling) pool of 1600–4800 vesicles and a synapsin-dependent (reserve) pool of 2400–7200 vesicles. The exact time course of vesicle recycling has not been measured. Clathrin-mediated endocytosis occur 40–120 s following prolonged stimulation. Activity-dependent bulk endocytosis also occur. Kiss-and-run has been suggested, but direct morphological evidence is lacking.
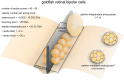
Figure 5
Vesicle pools and endocytic pathways at goldfish bipolar neuron terminals. The axonal terminals of goldfish bipolar neurons possess 45–65 active zones. At each active zone, there is a large electron-dense body known as the synaptic ribbon. Twenty to 30 vesicles are docked at the base of the synaptic ribbon and are readily releasable. Another 80 or so are tethered to the ribbon; these vesicles belong to the recycling pool. Nine-thousand to 16,000 vesicles per active zone are in the reserve pool. Two endocytic pathways exist at these terminals: a fast pathway (_τ_fast ≈ 2 s) following weak stimulation and a slow pathway (_τ_slow ≈ 20 s with delayed initiation) following repeated strong stimulation. The fast pathway is likely operated by clathrin-independent endocytosis (potentially ultrafast endocytosis or fast compensatory endocytosis), while the slow pathway could represent either clathrin-mediated endocytosis or activity-dependent bulk endocytosis.
Figure 6
Vesicle pools and endocytic pathways at the calyx of Held. A typical presynaptic bouton of the calyx of Held contains ~550 active zones. At each active zone, 4–10 vesicle constitute the RRP and can fuse in response to a single 20 ms depolarizing pulse. An additional 10–16 vesicles are utilized following 5 min stimulation at 2 Hz, and therefore belong to the recycling pool. Roughly 320 vesicles per active zone are in the reserve pool. Three endocytic pathways exist at the calyx of Held: a fast pathway (_τ_fast ≈ 1–2 s) triggered by single or brief trains of action potentials, a slow pathway (_τ_slow ≈ 12–23 s) triggered by 20 ms depolarizing pulses, and an additional fast pathway (_τ_fast ≈ 1.1–2 s) triggered by repeated 20 ms pulses or high-frequency action potential trains. The fast pathway triggered by single action potentials likely represents ultrafast endocytosis. The slow pathway is likely operated by activity-dependent bulk endocytosis and clathrin-mediated endocytosis. The additional fast pathway induced by intense stimulation might be a dynamin-independent and actin-dependent form of bulk endocytosis.
Figure 7
Vesicle pools and endocytic pathways at rodent hippocampal synapses. A conventional small synapse in the hippocampus contains a single active zone, at which about 10 vesicles are docked. These vesicles are in the RRP. An additional 16 vesicles are in the recycling pool, while ~240 are in the reserve pool. Synaptic vesicle recycling occurs on a timescale of 7–10 s at physiological temperatures as measured by optical imaging. This time constant matches the time constant for regeneration of synaptic vesicles through ultrafast endocytosis and subsequent endosomal sorting, but traditionally this pathway is thought to be operated by clathrin-mediated endocytosis. Activity bulk endocytosis occurs in response to intense stimulation and is completed over several minutes. Kiss-and-run has also been proposed but is still debated.
Similar articles
- Synaptic vesicle recycling at the calyx of Held.
Xue L, Mei YA. Xue L, et al. Acta Pharmacol Sin. 2011 Mar;32(3):280-7. doi: 10.1038/aps.2010.212. Epub 2011 Jan 24. Acta Pharmacol Sin. 2011. PMID: 21258358 Free PMC article. Review. - Endocytosis at ribbon synapses.
LoGiudice L, Matthews G. LoGiudice L, et al. Traffic. 2007 Sep;8(9):1123-8. doi: 10.1111/j.1600-0854.2007.00591.x. Epub 2007 Jun 5. Traffic. 2007. PMID: 17547701 Review. - The debate on the kiss-and-run fusion at synapses.
He L, Wu LG. He L, et al. Trends Neurosci. 2007 Sep;30(9):447-55. doi: 10.1016/j.tins.2007.06.012. Epub 2007 Aug 31. Trends Neurosci. 2007. PMID: 17765328 Review. - Evidence for a Clathrin-independent mode of endocytosis at a continuously active sensory synapse.
Fuchs M, Brandstätter JH, Regus-Leidig H. Fuchs M, et al. Front Cell Neurosci. 2014 Feb 25;8:60. doi: 10.3389/fncel.2014.00060. eCollection 2014. Front Cell Neurosci. 2014. PMID: 24616664 Free PMC article. - TRP Channel Trafficking.
Planells-Cases R, Ferrer-Montiel A. Planells-Cases R, et al. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. In: Liedtke WB, Heller S, editors. TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades. Boca Raton (FL): CRC Press/Taylor & Francis; 2007. Chapter 23. PMID: 21204515 Free Books & Documents. Review.
Cited by
- Control of Synapse Structure and Function by Actin and Its Regulators.
Gentile JE, Carrizales MG, Koleske AJ. Gentile JE, et al. Cells. 2022 Feb 9;11(4):603. doi: 10.3390/cells11040603. Cells. 2022. PMID: 35203254 Free PMC article. Review. - The Synaptic Vesicle Cycle Revisited: New Insights into the Modes and Mechanisms.
Chanaday NL, Cousin MA, Milosevic I, Watanabe S, Morgan JR. Chanaday NL, et al. J Neurosci. 2019 Oct 16;39(42):8209-8216. doi: 10.1523/JNEUROSCI.1158-19.2019. J Neurosci. 2019. PMID: 31619489 Free PMC article. Review. - Vesicle and reaction-diffusion hybrid modeling with STEPS.
Hepburn I, Lallouette J, Chen W, Gallimore AR, Nagasawa-Soeda SY, De Schutter E. Hepburn I, et al. Commun Biol. 2024 May 15;7(1):573. doi: 10.1038/s42003-024-06276-5. Commun Biol. 2024. PMID: 38750123 Free PMC article. - Quantitative Synaptic Biology: A Perspective on Techniques, Numbers and Expectations.
Reshetniak S, Fernández-Busnadiego R, Müller M, Rizzoli SO, Tetzlaff C. Reshetniak S, et al. Int J Mol Sci. 2020 Oct 2;21(19):7298. doi: 10.3390/ijms21197298. Int J Mol Sci. 2020. PMID: 33023247 Free PMC article. Review. - Constitutive Plasma Membrane Turnover in T-REx293 cells via Ordered Membrane Domain Endocytosis under Mitochondrial Control.
Deisl C, Moe OW, Hilgemann DW. Deisl C, et al. bioRxiv [Preprint]. 2024 Feb 14:2024.01.17.576124. doi: 10.1101/2024.01.17.576124. bioRxiv. 2024. PMID: 38293164 Free PMC article. Preprint.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases