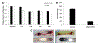Achieving Long-Term Biocompatible Silicone via Covalently Immobilized S-Nitroso- N-acetylpenicillamine (SNAP) That Exhibits 4 Months of Sustained Nitric Oxide Release - PubMed (original) (raw)
Achieving Long-Term Biocompatible Silicone via Covalently Immobilized S-Nitroso- N-acetylpenicillamine (SNAP) That Exhibits 4 Months of Sustained Nitric Oxide Release
Sean P Hopkins et al. ACS Appl Mater Interfaces. 2018.
Abstract
Ever since the role of endogenous nitric oxide (NO) in controlling a wide variety of biological functions was discovered approximately three decades back, multiple NO-releasing polymeric materials have been developed. However, most of these materials are typically short lived due to the inefficient incorporation of the NO donor molecules within the polymer matrix. In the present study, S-nitroso- N-acetyl penicillamine (SNAP) is covalently attached to poly(dimethylsiloxane) (PDMS) to create a highly stable nitric oxide (NO) releasing material for biomedical applications. By tethering SNAP to the cross-linker of PDMS, the NO donor is unable to leach into the surrounding environment. This is the first time that a sustainable NO release and bacterial inhibition for over 125 days has been achieved by any NO-releasing polymer with supporting evidence of potential long-term hemocompatibility and biocompatibility. The material proves to have very high antibacterial efficacy against Staphylococcus aureus by demonstrating a 99.99% reduction in the first 3 days in a continuous flow CDC bioreactor, whereas a similar inhibitory potential of 99.50% was maintained by the end of 1 month. Hemocompatibility of SNAP-PDMS was tested using a rabbit extracorporeal circuit (ECC) model over a 4 h period. Thrombus formation was greatly reduced within the SNAP-PDMS-coated ECCs compared to the control circuits, observing a 78% reduction in overall thrombus mass accumulation. These results demonstrate the potential of utilizing this material for blood and tissue contacting biomedical devices in long-term clinical applications where infection and unwanted clotting are major issues.
Keywords: S-nitroso-N-acetyl penicillamine; antimicrobial; bioreactor; extracorporeal circulation; hemocompatibility; nitric oxide.
Figures
Figure 1.
Synthesis route for covalently binding the SNAP molecule to hydroxy terminated PDMS polymer.
Figure 2.
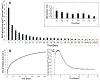
Nitric oxide releasing kinetics of SNAP-PDMS films where (A) continuous NO release flux measurements were taken on specified days while storing the films in PBS with EDTA at 37 °C (n=4). The green line represents the minimum physiological level of NO flux (0.5 × 10−10 mol cm−2 min−1). (B) Cumulative NO release over the 125-day testing period was measured and normalized per cm2 of SNAP-PDMS. (C) Representative NO release profile on day 0 from SNAP-PDMS films when placed in PBS with EDTA at 37 °C. Error bars represent standard deviation.
Figure 3.
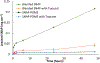
Cumulative leaching of SNAP into PBS from SNAP blended PDMS and covalent bound SNAP-PDMS films with and without a topcoat over the course of 48 hours (n=3). P<0.05 were used for comparison. Error bars represent standard deviation.
Figure 4.
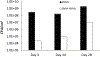
Long term antimicrobial ability of S. aureus adhesion to SNAP-PDMS. (A) Bacterial adhesion in 28-day bioreactor study on control PDMS and SNAP-PDMS films (n=3 per timepoint), showing approximately a 4, 3, and 2 log reduction in bacterial adhesion by days 3, 14, and 28 respectively. P<0.05 was used for comparison between groups. Error bars represent standard deviation.
Figure 5.
Films previously tested for 125 days under physiological conditions were also examined (n=3), still demonstrating a 58.6% reduction in viable bacteria. Error bars represent standard deviation.
Figure 6.
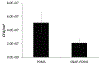
Hemocompatibility measurements of SNAP-PDMS coated tubing for ECC testing. (A) Time-dependent effects of NO release from the ECC on platelet count over the course of the 4 h study (n=3). (B) Quantification of clot mass obtained from the thrombogenicity chamber. (C) Visual representation of the clotting that occurred in PDMS coated controls (left) and SNAP-PDMS coated circuits (right). P<0.05 was used for comparison. Error bars represent standard deviation.
Similar articles
- Improved hemocompatibility of silicone rubber extracorporeal tubing via solvent swelling-impregnation of S-nitroso-N-acetylpenicillamine (SNAP) and evaluation in rabbit thrombogenicity model.
Brisbois EJ, Major TC, Goudie MJ, Bartlett RH, Meyerhoff ME, Handa H. Brisbois EJ, et al. Acta Biomater. 2016 Jun;37:111-9. doi: 10.1016/j.actbio.2016.04.025. Epub 2016 Apr 16. Acta Biomater. 2016. PMID: 27095484 Free PMC article. - Improved Hemocompatibility of Multilumen Catheters via Nitric Oxide (NO) Release from S-Nitroso-N-acetylpenicillamine (SNAP) Composite Filled Lumen.
Brisbois EJ, Kim M, Wang X, Mohammed A, Major TC, Wu J, Brownstein J, Xi C, Handa H, Bartlett RH, Meyerhoff ME. Brisbois EJ, et al. ACS Appl Mater Interfaces. 2016 Nov 2;8(43):29270-29279. doi: 10.1021/acsami.6b08707. Epub 2016 Oct 21. ACS Appl Mater Interfaces. 2016. PMID: 27734679 Free PMC article. - Origin of Long-Term Storage Stability and Nitric Oxide Release Behavior of CarboSil Polymer Doped with S-Nitroso-N-acetyl-D-penicillamine.
Wo Y, Li Z, Brisbois EJ, Colletta A, Wu J, Major TC, Xi C, Bartlett RH, Matzger AJ, Meyerhoff ME. Wo Y, et al. ACS Appl Mater Interfaces. 2015 Oct 14;7(40):22218-27. doi: 10.1021/acsami.5b07501. Epub 2015 Oct 1. ACS Appl Mater Interfaces. 2015. PMID: 26393943 Free PMC article. - Recent advances in thromboresistant and antimicrobial polymers for biomedical applications: just say yes to nitric oxide (NO).
Wo Y, Brisbois EJ, Bartlett RH, Meyerhoff ME. Wo Y, et al. Biomater Sci. 2016 Aug 19;4(8):1161-83. doi: 10.1039/c6bm00271d. Epub 2016 May 26. Biomater Sci. 2016. PMID: 27226170 Free PMC article. Review. - SNAP@CQD as a promising therapeutic vehicle against HCoVs: An overview.
Chatterjee S, Chakraborty A, Banik J, Mahindru S, Sharma AK, Mukherjee M. Chatterjee S, et al. Drug Discov Today. 2023 Jul;28(7):103601. doi: 10.1016/j.drudis.2023.103601. Epub 2023 Apr 28. Drug Discov Today. 2023. PMID: 37119964 Free PMC article. Review.
Cited by
- Development of Novel Amphotericin B-Immobilized Nitric Oxide-Releasing Platform for the Prevention of Broad-Spectrum Infections and Thrombosis.
Devine R, Douglass M, Ashcraft M, Tayag N, Handa H. Devine R, et al. ACS Appl Mater Interfaces. 2021 May 5;13(17):19613-19624. doi: 10.1021/acsami.1c01330. Epub 2021 Apr 27. ACS Appl Mater Interfaces. 2021. PMID: 33904311 Free PMC article. - Engineering Nitric Oxide-Releasing Antimicrobial Dental Coating for Targeted Gingival Therapy.
Chug MK, Crutchfield N, Garren M, Handa H, Brisbois EJ. Chug MK, et al. ACS Appl Bio Mater. 2024 May 20;7(5):2993-3004. doi: 10.1021/acsabm.4c00051. Epub 2024 Apr 9. ACS Appl Bio Mater. 2024. PMID: 38593411 Free PMC article. - Nitric oxide releasing halloysite nanotubes for biomedical applications.
Ghalei S, Hopkins S, Douglass M, Garren M, Mondal A, Handa H. Ghalei S, et al. J Colloid Interface Sci. 2021 May 15;590:277-289. doi: 10.1016/j.jcis.2021.01.047. Epub 2021 Jan 21. J Colloid Interface Sci. 2021. PMID: 33548611 Free PMC article. - Long-Term Storage Stability and Nitric Oxide Release Behavior of (_N_-Acetyl-_S_-nitrosopenicillaminyl)-_S_-nitrosopenicillamine-Incorporated Silicone Rubber Coatings.
Kumar R, Chug MK, Brisbois EJ. Kumar R, et al. ACS Appl Mater Interfaces. 2022 Jul 13;14(27):30595-30606. doi: 10.1021/acsami.2c06712. Epub 2022 Jun 27. ACS Appl Mater Interfaces. 2022. PMID: 35759508 Free PMC article. - Toward an artificial endothelium: Development of blood-compatible surfaces for extracorporeal life support.
Roberts TR, Garren MRS, Handa H, Batchinsky AI. Roberts TR, et al. J Trauma Acute Care Surg. 2020 Aug;89(2S Suppl 2):S59-S68. doi: 10.1097/TA.0000000000002700. J Trauma Acute Care Surg. 2020. PMID: 32251267 Free PMC article.
References
- Gorbet MB; Sefton MV, Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. Biomaterials 2004, 25 (26), 5681–5703. - PubMed
- MacMicking J; Xie; Nathan C, Nitric Oxide and Macrophage Function. Annu. Rev. Immunol 1997, 15 (1), 323–350. - PubMed
- Frost MC; Reynolds MM; Meyerhoff ME, Polymers Incorporating Nitric Oxide Releasing/Generating Substances for Improved Biocompatibility of Blood-Contacting Medical Devices. Biomaterials 2005, 26 (14), 1685–1693. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources