Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids - PubMed (original) (raw)
Lactobacillus rhamnosus GG prevents epithelial barrier dysfunction induced by interferon-gamma and fecal supernatants from irritable bowel syndrome patients in human intestinal enteroids and colonoids
Xu Han et al. Gut Microbes. 2019.
Abstract
Disruption of intestinal barrier homeostasis is an important pathogenic factor in conditions such as irritable bowel syndrome (IBS). Lactobacillus rhamnosus GG (LGG) improves IBS symptoms through unclear mechanisms. Previous studies utilizing colorectal adenocarcinoma cell lines showed that LGG metabolites prevented interferon gamma (IFN-gamma) induced barrier damage but the model employed limited these findings. We aimed to interrogate the protective effects of LGG on epithelial barrier function using human intestinal epithelial cultures (enteroids and colonoids) as a more physiologic model. To investigate how LGG affects epithelial barrier function, we measured FITC-Dextran (FD4) flux across the epithelium as well as tight junction zonula occludens 1 (ZO-1) and occludin (OCLN) expression. Colonoids were incubated with fecal supernatants from IBS patients (IBS-FSN) and healthy controls in the presence or absence of LGG to examine changes in gut permeability. Enteroids incubated with IFN-gamma demonstrated a downregulation of OCLN and ZO-1 expression by 67% and 50%, respectively (p<0.05). This was accompanied by increased paracellular permeability as shown by leakage of FD4. Pretreatment of enteroids with LGG prevented these changes and normalized OCLN and ZO-1 to control levels. These actions were independent of its action against apoptosis. However, these protective effects were not seen with LGG cell wall extracts, LGG DNA, or denatured (boiled) LGG. Intriguingly, IBS-FSN injected into colonoids increased paracellular permeability, which was prevented by LGG. LGG, likely due to secreted proteins, protects against epithelial barrier dysfunction. Bacterial-derived factors to modulate gut barrier function may be a treatment option in disorders such as IBS.
Keywords: IFN-gamma; Lactobacillus rhamnosus GG metabolites; epithelial barrier function; human colonoids; human intestinal enteroids; irritable bowel syndrome; tight junction.
Figures
Figure 1.
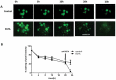
Human Intestinal Enteroids Have An Intact Intestinal Epithelium That Can Be Modified By EGTA (A) Representative images of FD4 dynamics in human enteroids. Human enteroids were injected with FD4 and imaged at 0, 5, 10, 20, and 23h postinjection. Control group retained the majority of injected FD4 within the lumen over 12 h. The addition of EGTA to the media 20h after injection resulted in the rapid loss of FD4 from the lumen, indicating the loss of epithelial paracellular barrier function. (B) Quantitation of barrier disruption by determination of the fraction of initial FD4 fluorescence retained over time. Points represent the medians, and bars represent the interquartile ranges. Control human enteroids retained 55% of FD4 over 20h, while the EGTA-treated enteroids retained 7% of FD4 over 20h (p < 0.01* by Mann-Whitney test, compared to control).
Figure 2.
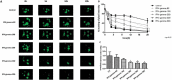
IFN-gamma disrupts intestinal epithelial barrier function and reduces gene expression of ZO-1 in a concentration-dependent manner (A) Representative images of FD4 leakage from the lumen of human enteroids treated with IFN-gamma compared with human enteroids under control conditions. The human enteroids were injected with the fluorescence dye FD4 and then exposed to IFN-gamma at 60, 100, 200, 300, and 500 ng/ml for 20h. (B) Quantitation of retention of FD4 fluorescence in human enteroids relative to time zero. The highest dose of IFN-gamma applied (500 ng/ml) resulted in a rapid loss with 20% retention of fluorescence by 3h after treatment, and 4% of fluorescence remaining at 20h. Meanwhile, the lowest dose of IFN-gamma (60 ng/ml) caused 70% loss of the fluorescence by 20h (*p<0.05, compared to control). (C) Treatment of human enteroids with IFN-gamma caused a dose dependent decrease of ZO-1 gene expression by qPCR.
Figure 3.
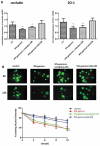
LGG specifically protects against human enteroid barrier dysfunction induced by IFN-gamma (A) Human enteroids were pretreated with LGG-CM or L. crispatus-CM overnight, and then they were exposed to IFN-gamma (200 ng/ml) for 24h. LGG-CM prevented IFN-gamma-induced downregulation of occludin and ZO-1 gene expression. However, this protective effect was not seen in enteroids incubated with L. Crispatus (*p<0.05, compared to control, CT). (B) Similarly, LGG-CM, but not L. Crispatus-CM, prevented leakage of the fluorescent dye FD4 induced by IFN-gamma. Under control conditions, the human enteroids retained 76% of FD4 at 12h. Treatment of the enteroids with IFN-gamma led to intestinal epithelial barrier dysfunction with only 35% of the dye retained at 12h. Administration of LGG, but not L. crispatus-CM, prevented leakage of dye evoked by IFN-gamma (*p<0.05, compared to control).
Figure 4.
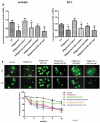
Protein metabolites of LGG prevent IFN-gamma-induced downregulation of tight junction gene expression and epithelial barrier dysfunction in human enteroids (A) Incubation of human enteroids with IFN-gamma (200 ng/ml) caused a 67% and 50% downregulation of gene expression of occludin and ZO-1, respectively (P<0.05). Pretreatment of enteroids with LGG-CM prevented these changes and normalized occludin and ZO-1 to control levels. In contrast, addition of LGG extracted DNA, boiled LGG-CM, or LGG cell wall led to expression of occludin and ZO-1 comparable to enteroids exposed to IFN-gamma (*p<0.05, compared to control, CT). (B) Under control conditions, the enteroids retained 70% of FD4 at 12 h, while treatment of the enteroids with IFN-gamma resulted in 30% retention of dye at 12 h. Administration of LGG-CM prevented leakage of dye induced by IFN-gamma. In contrast, LGG DNA, cell wall or boiled supernatant did not protect against barrier dysfunction evoked by IFN-gamma.
Figure 5.
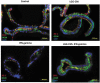
Lactobacillus rhamnosus GG prevents downregulation of ZO-1 and occludin expression induced by IFN-gamma Human enteroids were pretreated with or without LGG-CM overnight, and then exposed to IFN-gamma (200 ng/ml). After treatment with IFN-gamma for 20 h, the human enteroids were fixed and stained. ZO-1 is present at the tight junction near the apical surface of the epithelium, whereas occludin stained in green is seen at the tight junction and along the lateral surface of the cell in control human enteroids. In contrast, human enteroids treated with IFN-gamma, apical ZO-1 at the tight junction is lost and occludin is no longer restricted to the lateral surface of the epithelial cell. Meanwhile, pretreatment of human enteroids with LGG-CM have immunofluorescence for ZO-1 and occludin similar to those of the control.
Figure 6.
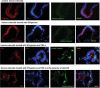
Protective effects of LGG-CM occur in the absence of apoptosis. Human enteroids were treated with IFN-gamma (200 ng/ml), or a “cytokine mixture” containing TNF-a (1000 ng/ml) and IFN-gamma (1000ng/ml) for 24 h in the presence or absence of 12h pretreatment with LGG-CM. Immunofluorescence study showed that expression of cleaved caspase-3 (green fluorescence) was absent in enteroids exposed to IFN-gamma at 200ng/ml and was similar to those of the control enteroids. Treatment with the cytokine mixture containing TNF-a and INF-gamma induced Caspase-3 activation. Immunofluorescence staining of enteroids treated with IFN-gamma in the presence of LGG-CM was not shown since caspase-3 immunoreactivities were not observed and were similar to control and treatment with IFN-gamma in the absence of LGG-CM. Cell death was found in enteroids treated with TNF-a and INF-gamma. Pretreatment with LGG-CM did not prevent apoptosis induced by the cytokine mixture IFN-gamma plus TNF-a.
Figure 7.
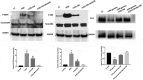
Protective actions of LGG on junction proteins were mediated by pathways independent of MAPK/ERK signaling cascade in human colonoids. Human colonoids were treated with LGG-CM or EGF (100 ng/ml) for 90 min in the presence or absence of 1-h pretreatment with an EGFR inhibitor, AG1478 (200nM). In separate experiments, human colonoids were treated with IFN-gamma (200 ng/ml) in the absence or presence of LGG. The protective action of LGG was examined in the presence of AG 1478 (200nM). Cellular lysates were prepared and immunoblotted for P-EGFR, total EGFR, P-ERK, total ERK, occludin, ZO-1 and GAPDH. Bands of GAPDH were used as control for an equal protein loading. The optical density is expressed in arbitrary units normalized against a control sample. Data in histograms represent means ± SE; n = 5 in each group (control group vs EGF group *p<0.05, control group vs LGG group #p<0.05, control group vs IFN-gamma group, §p<0.05).
Figure 8.
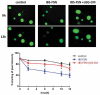
LGG-CM prevents barrier dysfunction in human colonoids induced by fecal supernatants from IBS-D patients. Fecal supernatants (FSN) from IBS patients (n = 4) were used to induce epithelial barrier damage. Human colonoids were incubated for 12 hr with FSN from healthy subjects (n = 4) and IBS patients (n = 4) with and without cell-free LGG supernatant. In the presence of FSN from healthy subjects, the colonoids retained 80% of FD4 at 12 hr. Treatment of the colonoids with IBS-FSN impaired permeability resulting in 39% retention of dye after 12 hr. Administration of LGG supernatant prevented leakage of dye evoked by IBS-FSN. * p<0.05 compared to control
Similar articles
- Multispecies probiotic protects gut barrier function in experimental models.
Nébot-Vivinus M, Harkat C, Bzioueche H, Cartier C, Plichon-Dainese R, Moussa L, Eutamene H, Pishvaie D, Holowacz S, Seyrig C, Piche T, Theodorou V. Nébot-Vivinus M, et al. World J Gastroenterol. 2014 Jun 14;20(22):6832-43. doi: 10.3748/wjg.v20.i22.6832. World J Gastroenterol. 2014. PMID: 24944474 Free PMC article. - The JAK-Inhibitor Tofacitinib Rescues Human Intestinal Epithelial Cells and Colonoids from Cytokine-Induced Barrier Dysfunction.
Sayoc-Becerra A, Krishnan M, Fan S, Jimenez J, Hernandez R, Gibson K, Preciado R, Butt G, McCole DF. Sayoc-Becerra A, et al. Inflamm Bowel Dis. 2020 Feb 11;26(3):407-422. doi: 10.1093/ibd/izz266. Inflamm Bowel Dis. 2020. PMID: 31751457 Free PMC article. - Lactobacillus rhamnosus GG attenuates interferon-{gamma} and tumour necrosis factor-alpha-induced barrier dysfunction and pro-inflammatory signalling.
Donato KA, Gareau MG, Wang YJJ, Sherman PM. Donato KA, et al. Microbiology (Reading). 2010 Nov;156(Pt 11):3288-3297. doi: 10.1099/mic.0.040139-0. Epub 2010 Jul 23. Microbiology (Reading). 2010. PMID: 20656777 - Tight junctions and IBS--the link between epithelial permeability, low-grade inflammation, and symptom generation?
Piche T. Piche T. Neurogastroenterol Motil. 2014 Mar;26(3):296-302. doi: 10.1111/nmo.12315. Neurogastroenterol Motil. 2014. PMID: 24548256 Review. - Effect of lactobacilli on paracellular permeability in the gut.
Ahrne S, Hagslatt ML. Ahrne S, et al. Nutrients. 2011 Jan;3(1):104-17. doi: 10.3390/nu3010104. Epub 2011 Jan 12. Nutrients. 2011. PMID: 22254077 Free PMC article. Review.
Cited by
- Organoid Models of Colorectal Pathology: Do They Hold the Key to Personalized Medicine? A Systematic Review.
DeHaan RK, Sarvestani SK, Huang EH. DeHaan RK, et al. Dis Colon Rectum. 2020 Nov;63(11):1559-1569. doi: 10.1097/DCR.0000000000001806. Dis Colon Rectum. 2020. PMID: 32868555 Free PMC article. - Crosstalk Between the Gut Microbiota and Epithelial Cells Under Physiological and Infectious Conditions.
Zhou A, Yuan Y, Yang M, Huang Y, Li X, Li S, Yang S, Tang B. Zhou A, et al. Front Cell Infect Microbiol. 2022 Jan 27;12:832672. doi: 10.3389/fcimb.2022.832672. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35155283 Free PMC article. Review. - Effects of different probiotic strains B. lactis, L. rhamnosus and L. reuteri on brain-intestinal axis immunomodulation in an endotoxin-induced inflammation.
Michels M, Jesus GFA, Abatti MR, Córneo E, Cucker L, de Medeiros Borges H, da Silva Matos N, Rocha LB, Dias R, Simon CS, Voytena APL, Rossetto M, Ramlov F, Dal-Pizzol F. Michels M, et al. Mol Neurobiol. 2022 Aug;59(8):5168-5178. doi: 10.1007/s12035-022-02906-3. Epub 2022 Jun 8. Mol Neurobiol. 2022. PMID: 35674863 - Role of Intestinal Microbes in Chronic Liver Diseases.
Xu M, Luo K, Li J, Li Y, Zhang Y, Yuan Z, Xu Q, Wu X. Xu M, et al. Int J Mol Sci. 2022 Oct 21;23(20):12661. doi: 10.3390/ijms232012661. Int J Mol Sci. 2022. PMID: 36293518 Free PMC article. Review. - The potential role of adherence factors in probiotic function in the gastrointestinal tract of adults and pediatrics: a narrative review of experimental and human studies.
Gorreja F, Walker WA. Gorreja F, et al. Gut Microbes. 2022 Jan-Dec;14(1):2149214. doi: 10.1080/19490976.2022.2149214. Gut Microbes. 2022. PMID: 36469568 Free PMC article. Review.
References
- Mullin JM, Laughlin KV, Marano CW, Russo LM, Soler AP.. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. Am J Physiol. 1992;263:F915–924.PMID:1279987 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK110436/DK/NIDDK NIH HHS/United States
- U19 AI116482/AI/NIAID NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- U01 DK103141/DK/NIDDK NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- R01 DK048419/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous