Rapid Endothelialization of Off-the-Shelf Small Diameter Silk Vascular Grafts - PubMed (original) (raw)
Rapid Endothelialization of Off-the-Shelf Small Diameter Silk Vascular Grafts
Elysse C Filipe et al. JACC Basic Transl Sci. 2018.
Abstract
Synthetic vascular grafts for small diameter revascularization are lacking. Clinically available conduits expanded polytetrafluorethylene and Dacron fail acutely due to thrombosis and in the longer term from neointimal hyperplasia. We report the bioengineering of a cell-free, silk-based vascular graft. In vitro we demonstrate strong, elastic silk conduits that support rapid endothelial cell attachment and spreading while simultaneously resisting blood clot and fibrin network formation. In vivo rat studies show complete graft patency at all time points, rapid endothelialization, and stabilization and contraction of neointimal hyperplasia. These studies show the potential of silk as an off-the-shelf small diameter vascular graft.
Keywords: ECM, extracellular matrix; PCL, polycaprolactone; PCNA, proliferating cell nuclear antigen; SMC-α, smooth muscle cell actin; ePTFE, expanded polytetrafluoroethylene; rat aortic interposition; silk; small diameter; vWF, von Willebrand factor; vascular graft.
Figures
Graphical abstract
Figure 1
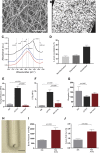
Mechanical Properties of Electrospun Silk Scaffolds for Vascular Grafting (A) Scanning electron micrograph of electrospun silk. Scale bar = 10 μm. (B) Cross section of electrospun silk (white), demonstrating scaffold porosity (black). (C) Representative amine I Fourier transform infrared spectroscopy spectra of silk prior to electrospinning (red), post-electrospinning (blue), and after cross-linking (black). (D) Proportion of β-sheet crystallinity in electrospun silk prior to electrospinning, post-electrospinning, and after cross-linking. Mechanical properties of silk, compared with expanded polytetrafluoroethylene (ePTFE) and fresh rat aorta as control scaffolds. (E) Youngs moduli, (F) ultimate tensile strength (UTS), and (G) percentage of elongation. Data are mean ± SEM; n = 9 to 12 (n = 2 for rat aorta). (H) Macroscopic images of electrospun silk graft. Scale bar = 1 mm. (I) Quantification of the maximum pressure before failure. Data are mean ± SEM; n = 6 (n = 3 for rat aorta). (J) Suture pull-out data of silk graft, with rat aorta as a control. Data are mean ± SEM; n = 7 (n = 3 for rat aorta).
Figure 2
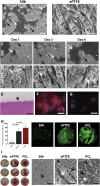
Endothelial Cell and Blood Compatibility With Electrospun Silk Scaffolds for Vascular Grafting Representative scanning electron micrograph images of endothelial cell attachment on electrospun silk scaffolds (A) and expanded polytetrafluoroethylene (ePTFE) control scaffolds (B). Scale bar = 20 μm. Representative scanning electron micrograph images of endothelial cells on electrospun silk (C) and ePTFE scaffolds (D) at 1, 3, and 6 days post-seeding. Scale bar = 20 μm. (A to D) White arrows demonstrate cells and black arrows show scaffold surface. (E) Representative cross-sectional image of an endothelial cell monolayer on the surface of electrospun silk. Scale bar = 50 μm. vascular endothelial cadherin staining of endothelial cells on the surface of electrospun silk (F) and ePTFE (G). Scale bar = 25 μm. (H) Quantification of fluorescent fibrinogen on silk, polycaprolactone (PCL), and ePTFE scaffolds. Representative images of the scaffolds demonstrating fibrin network formation in green. (I) Macroscopic images of the whole blood assay on silk, PCL, and ePTFE scaffolds. Representative scanning electron micrograph images of these same scaffolds revealing red blood cells (black arrows) and fibrin deposition (white arrows) on the different scaffolds. Scale bar = 25 μm.
Figure 3
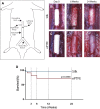
In Vivo Experimental Design in a Rat Model of Abdominal Aortic Replacement (A) Schematic of in vivo implantation procedure in rat. (B) Silk and (C) expanded polytetrafluoroethylene (ePTFE) control grafts at implantation and at the explantation time points of 6 and 24 weeks. (D) Kaplan-Meier curve depicting graft survival of silk (blue) and ePTFE (red). n = 21 and 22 for silk and ePTFE grafts, respectively.
Figure 4
Scanning Electron Microscopy of Silk and ePTFE Grafts at Explantation Scanning electron micrograph images of explanted silk (A) and expanded polytetrafluoroethylene (ePTFE) (C) grafts at 3, 6, 12, and 24 weeks. Scale bar = 500 μm. Representative high magnification images (black boxes in A and C) of silk (B) and ePTFE (D). Scale bar = 50 μm.
Figure 5
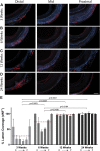
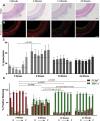
Immunohistologic Staining for vWF+ Cells in Silk Grafts at Explantation Representative micrograph images of von Willebrand factor (vWF) immunohistochemistry staining on cross sections of silk grafts at 3 (A), 6 (B), 12 (C), and 24 (D) weeks. vWF staining is red, and 4′,6-diamidino-2-phenylindole staining is blue. Scale bar = 200 μm. (E) Quantification of vWF coverage on the lumen of the graft depicting vWF positivity as early as 3 weeks and complete coverage by 12 weeks. N = 4 animals per time point with 3 images analyzed per region per graft. The red dashed line indicates the average vWF+ staining for each time point. Statistical analysis was performed on these values. D → P indicates selected equidistant regions within the graft, from distal to proximal.
Figure 6
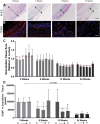
Histologic Assessment of Neointima Formation and SMC Phenotype in Silk Grafts (A) Representative micrograph images of hematoxylin and eosin (H&E) staining of silk grafts at all time points. Scale bar = 200 μm. (B) Representative fluorescent micrograph image of proliferating cell nuclear antigen (PCNA)/smooth muscle cell actin (SMC-α) double stain at all explantation time points. Scale bar = 200 μm. (C) Quantification of neointima, represented as a percentage of total lumen size for each graft. N = 4 animals per time point with 3 images analyzed per region per graft. (D) Quantification of positive PCNA (red) and SMC-α (green) staining, represented as a percentage of the total positive staining in each graft section. N = 4 animals per time point with 3 images analyzed per region per graft. The dashed lines indicate the average neointima values (C) and average PCNA and SMC-α actin staining (D) for each time point. Statistical analysis was performed on these values. D → P indicates selected equidistant regions within the graft, from distal to proximal.
Figure 7
Granulation Tissue Response and Immune Cell Accumulation in the Adventitia of Silk Grafts (A) Representative micrograph images of hematoxylin and eosin (H&E) staining of the adventitia surrounding the silk grafts at all time points. Scale bar = 100 μm. (B) Representative fluorescent micrograph images of CD68 immunostaining at all time points. Scale bar = 100 μm. (C) Quantification of granulation tissue area, represented as a percentage of total lumen size for each graft. N = 4 animals per time point with 3 images analyzed per region per graft. (D) Quantification of positive CD68 staining, indicating macrophage presence, in the granulation tissue area, represented as a percentage of total granulation tissue for each section. N = 4 animals per time point with 3 images analyzed per region per graft. CD68 staining is red, and 4′,6-diamidino-2-phenylindole staining is blue. Scale bar = 100 μm. The red dashed lines indicate the average granulation tissue thickness values (C) and average CD68 staining (D) for each time point. Statistical analysis was performed on these values. D → P indicates selected equidistant regions within the graft, from distal to proximal.
Similar articles
- Experimental noninferiority trial of synthetic small-caliber biodegradable versus stable vascular grafts.
Mugnai D, Tille JC, Mrówczyński W, de Valence S, Montet X, Möller M, Walpoth BH. Mugnai D, et al. J Thorac Cardiovasc Surg. 2013 Aug;146(2):400-7.e1. doi: 10.1016/j.jtcvs.2012.09.054. Epub 2012 Oct 23. J Thorac Cardiovasc Surg. 2013. PMID: 23098749 - Development of a decellularized human amniotic membrane-based electrospun vascular graft capable of rapid remodeling for small-diameter vascular applications.
Liu J, Chen D, Zhu X, Liu N, Zhang H, Tang R, Liu Z. Liu J, et al. Acta Biomater. 2022 Oct 15;152:144-156. doi: 10.1016/j.actbio.2022.09.009. Epub 2022 Sep 13. Acta Biomater. 2022. PMID: 36108966 - In vivo evaluation of biomimetic fluorosurfactant polymer-coated expanded polytetrafluoroethylene vascular grafts in a porcine carotid artery bypass model.
Bastijanic JM, Marchant RE, Kligman F, Allemang MT, Lakin RO, Kendrick D, Kashyap VS, Kottke-Marchant K. Bastijanic JM, et al. J Vasc Surg. 2016 Jun;63(6):1620-1630.e4. doi: 10.1016/j.jvs.2015.01.060. Epub 2015 Mar 28. J Vasc Surg. 2016. PMID: 25827964 Free PMC article. - In-vivo evaluation of silk fibroin small-diameter vascular grafts: state of art of preclinical studies and animal models.
Settembrini A, Buongiovanni G, Settembrini P, Alessandrino A, Freddi G, Vettor G, Martelli E. Settembrini A, et al. Front Surg. 2023 May 26;10:1090565. doi: 10.3389/fsurg.2023.1090565. eCollection 2023. Front Surg. 2023. PMID: 37304180 Free PMC article. Review. - Considerations in the Development of Small-Diameter Vascular Graft as an Alternative for Bypass and Reconstructive Surgeries: A Review.
Obiweluozor FO, Emechebe GA, Kim DW, Cho HJ, Park CH, Kim CS, Jeong IS. Obiweluozor FO, et al. Cardiovasc Eng Technol. 2020 Oct;11(5):495-521. doi: 10.1007/s13239-020-00482-y. Epub 2020 Aug 18. Cardiovasc Eng Technol. 2020. PMID: 32812139 Review.
Cited by
- Evaluation of small-diameter silk vascular grafts implanted in dogs.
Tanaka T, Tanaka R, Ogawa Y, Takagi Y, Sata M, Asakura T. Tanaka T, et al. JTCVS Open. 2021 Mar 4;6:148-156. doi: 10.1016/j.xjon.2021.02.008. eCollection 2021 Jun. JTCVS Open. 2021. PMID: 36003556 Free PMC article. - Application of decellularized vascular matrix in small-diameter vascular grafts.
Li Y, Zhou Y, Qiao W, Shi J, Qiu X, Dong N. Li Y, et al. Front Bioeng Biotechnol. 2023 Jan 6;10:1081233. doi: 10.3389/fbioe.2022.1081233. eCollection 2022. Front Bioeng Biotechnol. 2023. PMID: 36686240 Free PMC article. Review. - Development of a Novel Hierarchically Biofabricated Blood Vessel Mimic Decorated with Three Vascular Cell Populations for the Reconstruction of Small-Diameter Arteries.
Carrabba M, Fagnano M, Ghorbel MT, Rapetto F, Su B, De Maria C, Vozzi G, Biglino G, Perriman AW, Caputo M, Madeddu P. Carrabba M, et al. Adv Funct Mater. 2023 Nov 3;34(7):adfm.202300621. doi: 10.1002/adfm.202300621. eCollection 2024 Feb. Adv Funct Mater. 2023. PMID: 39257639 Free PMC article. - Steady-State Behavior and Endothelialization of a Silk-Based Small-Caliber Scaffold In Vivo Transplantation.
Li H, Wang Y, Sun X, Tian W, Xu J, Wang J. Li H, et al. Polymers (Basel). 2019 Aug 3;11(8):1303. doi: 10.3390/polym11081303. Polymers (Basel). 2019. PMID: 31382650 Free PMC article.
References
- Seifu D.G., Purnama A., Mequanint K., Mantovani D. Small-diameter vascular tissue engineering. Nat Rev Cardiol. 2013;10:410–421. - PubMed
- Dahl S.L., Kypson A.P., Lawson J.H. Readily available tissue-engineered vascular grafts. Sci Transl Med. 2011;3:68ra9. - PubMed
- L'Heureux N., Paquet S., Labbe R., Germain L., Auger F.A. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12:47–56. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous