Tolerance of preterm formula versus pasteurized donor human milk in very preterm infants: a randomized non-inferiority trial - PubMed (original) (raw)
Randomized Controlled Trial
Tolerance of preterm formula versus pasteurized donor human milk in very preterm infants: a randomized non-inferiority trial
Simonetta Costa et al. Ital J Pediatr. 2018.
Abstract
Background: Human milk (HM) is the best feeding for premature infants. When own mother's milk (OMM) is insufficient or unavailable, pasteurized donor human milk (PDHM) and preterm formula (PF) are the alternative nutritional sources, but the benefits of donor milk over formula are not defined. This study aimed to assess whether, in the absence of OMM, the PF could guarantee a feeding tolerance not inferior to that seen with the use of PDHM during the first two weeks of life of very preterm infants.
Methods: Infants with gestational age (GA) of ≤32 weeks who started enteral feeding within the first 7 days of life were randomized to receive PDHM or PF as a supplement to the OMM insufficient or unavailable. The primary outcome was the day of life when full enteral feeding (FEF) of 150 mL/Kg/d was achieved.
Results: Seventy infants were randomized, 35 in the PF group (GA 30.2 ± 1.7 weeks; BW 1342 ± 275 g), 35 in the PDHM group (GA 30 ± 1.9 weeks; BW 1365 ± 332 g). The time to achieve FEF was the same for infants fed with PF and for infants fed with PDHM (12.3 ± 7.0 days vs 12.8 ± 6.5).
Conclusions: This trial shows that PF could be a valid alternative for the early feeding of very preterm infants when OMM is insufficient or unavailable.
Trial registration: UMIN000013922 . Date of formal registration: December 31, 2014.
Keywords: Feeding tolerance; Pasteurized donor human milk; Preterm formula; Very preterm infant.
Conflict of interest statement
Ethics approval and consent to participate
The institutional review boards approved the study, and written informed consent was obtained from the parents of all subjects before enrollment.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
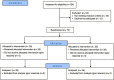
Fig. 1
Flow chart of the study
Similar articles
- Growth Benefits of Own Mother's Milk in Preterm Infants Fed Daily Individualized Fortified Human Milk.
de Halleux V, Pieltain C, Senterre T, Studzinski F, Kessen C, Rigo V, Rigo J. de Halleux V, et al. Nutrients. 2019 Apr 3;11(4):772. doi: 10.3390/nu11040772. Nutrients. 2019. PMID: 30987136 Free PMC article. - Effect of Donor Milk on Severe Infections and Mortality in Very Low-Birth-Weight Infants: The Early Nutrition Study Randomized Clinical Trial.
Corpeleijn WE, de Waard M, Christmann V, van Goudoever JB, Jansen-van der Weide MC, Kooi EM, Koper JF, Kouwenhoven SM, Lafeber HN, Mank E, van Toledo L, Vermeulen MJ, van Vliet I, van Zoeren-Grobben D. Corpeleijn WE, et al. JAMA Pediatr. 2016 Jul 1;170(7):654-61. doi: 10.1001/jamapediatrics.2016.0183. JAMA Pediatr. 2016. PMID: 27135598 Clinical Trial. - Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants.
Schanler RJ, Lau C, Hurst NM, Smith EO. Schanler RJ, et al. Pediatrics. 2005 Aug;116(2):400-6. doi: 10.1542/peds.2004-1974. Pediatrics. 2005. PMID: 16061595 Clinical Trial. - Probiotics and Time to Achieve Full Enteral Feeding in Human Milk-Fed and Formula-Fed Preterm Infants: Systematic Review and Meta-Analysis.
Aceti A, Gori D, Barone G, Callegari ML, Fantini MP, Indrio F, Maggio L, Meneghin F, Morelli L, Zuccotti G, Corvaglia L. Aceti A, et al. Nutrients. 2016 Jul 30;8(8):471. doi: 10.3390/nu8080471. Nutrients. 2016. PMID: 27483319 Free PMC article. Review. - Formula milk versus donor breast milk for feeding preterm or low birth weight infants.
Quigley MA, Henderson G, Anthony MY, McGuire W. Quigley MA, et al. Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002971. doi: 10.1002/14651858.CD002971.pub2. Cochrane Database Syst Rev. 2007. PMID: 17943776 Updated. Review.
Cited by
- [Clinical guidelines for the diagnosis and treatment of feeding intolerance in preterm infants (2020)].
Evidence-Based Medicine Group, Neonatologist Society, Chinese Medical Doctor Association. Evidence-Based Medicine Group, Neonatologist Society, Chinese Medical Doctor Association. Zhongguo Dang Dai Er Ke Za Zhi. 2020 Oct;22(10):1047-1055. doi: 10.7499/j.issn.1008-8830.2008132. Zhongguo Dang Dai Er Ke Za Zhi. 2020. PMID: 33059799 Free PMC article. Chinese. - Effects of Formula Milk Feeding in Premature Infants: A Systematic Review.
Moreira-Monteagudo M, Leirós-Rodríguez R, Marqués-Sánchez P. Moreira-Monteagudo M, et al. Children (Basel). 2022 Jan 24;9(2):150. doi: 10.3390/children9020150. Children (Basel). 2022. PMID: 35204871 Free PMC article. Review. - Efficacy of Donated Milk in Early Nutrition of Preterm Infants: A Meta-Analysis.
Li Y, Chi C, Li C, Song J, Song Z, Wang W, Sun J. Li Y, et al. Nutrients. 2022 Apr 21;14(9):1724. doi: 10.3390/nu14091724. Nutrients. 2022. PMID: 35565692 Free PMC article. - Characterization of exosomal microRNAs in preterm infants fed with breast milk and infant formula.
Kim EB, Song JH, Le LN, Kim H, Koh JW, Seo Y, Jeong HR, Kim HT, Ryu S. Kim EB, et al. Front Nutr. 2024 Jan 18;11:1339919. doi: 10.3389/fnut.2024.1339919. eCollection 2024. Front Nutr. 2024. PMID: 38304545 Free PMC article. - Unlocking the Potential: A Systematic Literature Review on the Impact of Donor Human Milk on Infant Health Outcomes.
Kashyap V, Choudhari SG. Kashyap V, et al. Cureus. 2024 Apr 2;16(4):e57440. doi: 10.7759/cureus.57440. eCollection 2024 Apr. Cureus. 2024. PMID: 38699095 Free PMC article. Review.
References
- Hallowell SG, Rogowski JA, Spatz DL, Hanlon AL, Kenny M, Lake ET. Factors associated with infant feeding of human milk at discharge from neonatal intensive care: cross-sectional analysis of nurse survey and infant outcomes data. Int J Nurs Stud. 2016;53:190. doi: 10.1016/j.ijnurstu.2015.09.016. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical