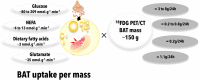Brown Adipose Tissue Energy Metabolism in Humans - PubMed (original) (raw)
Review
Brown Adipose Tissue Energy Metabolism in Humans
André C Carpentier et al. Front Endocrinol (Lausanne). 2018.
Abstract
The demonstration of metabolically active brown adipose tissue (BAT) in humans primarily using positron emission tomography coupled to computed tomography (PET/CT) with the glucose tracer 18-fluorodeoxyglucose (18FDG) has renewed the interest of the scientific and medical community in the possible role of BAT as a target for the prevention and treatment of obesity and type 2 diabetes (T2D). Here, we offer a comprehensive review of BAT energy metabolism in humans. Considerable advances in methods to measure BAT energy metabolism, including nonesterified fatty acids (NEFA), chylomicron-triglycerides (TG), oxygen, Krebs cycle rate, and intracellular TG have led to very good quantification of energy substrate metabolism per volume of active BAT in vivo. These studies have also shown that intracellular TG are likely the primary energy source of BAT upon activation by cold. Current estimates of BAT's contribution to energy expenditure range at the lower end of what would be potentially clinically relevant if chronically sustained. Yet, 18FDG PET/CT remains the gold-standard defining method to quantify total BAT volume of activity, used to calculate BAT's total energy expenditure. Unfortunately, BAT glucose metabolism better reflects BAT's insulin sensitivity and blood flow. It is now clear that most glucose taken up by BAT does not fuel mitochondrial oxidative metabolism and that BAT glucose uptake can therefore be disconnected from thermogenesis. Furthermore, BAT thermogenesis is efficiently recruited upon repeated cold exposure, doubling to tripling its total oxidative capacity, with reciprocal reduction of muscle thermogenesis. Recent data suggest that total BAT volume may be much larger than the typically observed 50-150 ml with 18FDG PET/CT. Therefore, the current estimates of total BAT thermogenesis, largely relying on total BAT volume using 18FDG PET/CT, may underestimate the true contribution of BAT to total energy expenditure. Quantification of the contribution of BAT to energy expenditure begs for the development of more integrated whole body in vivo methods.
Keywords: brown adipose tissue; energy metabolism; molecular imaging; obesity; positron emission tomography; tracer methods; type 2 diabetes.
Figures
Figure 1
Uncoupling protein 1 (UCP1)-mediated brown adipose tissue thermogenesis. Upper panel: Brown adipose tissue UCP1-mediated thermogenesis is activated by fatty acids produced via norepinephrine-induced intracellular triglyceride (TG) lipolysis during cold exposure. Middle panel: Acute pharmacological inhibition of intracellular TG lipolysis blunts brown adipose tissue thermogenesis via reduction of intracellular fatty acids availability. Lower panel: Genetic deletion-mediated inhibition of intracellular TG lipolysis in brown adipose tissues leads to increased reliance on circulating nonesterified fatty acids (NEFA) and triglyceride (TG)-rich lipoproteins to sustain UCP1-mediated thermogenesis. The mitochondrion illustration was obtained free of copyright from Pixabay (
, 2018).
Figure 2
The standard definition of brown adipose tissue in vivo in humans. Brown adipose tissue is currently defined in vivo in humans by the combination of two radiological features: (1) 18-fluorodeoxyglucose (18FDG) uptake above a set threshold higher than that usually observed in white adipose tissues using positron emission tomography (left panels); and (2) a radio-density that is compatible with the presence of adipose tissue using computed tomography (right panels). After intravenous (i.v.) injection of 18FDG, whole body (static) positron emission tomography scanning is performed, giving quantitative tissue bio-distribution of the tracer into brown adipose tissues. This tissue tracer uptake is co-registered with tissue radio-density measured using computed tomography. The middle left and right panels show positron emission tomography and computed tomography transverse views, respectively, of supraclavicular brown adipose tissue in a healthy individual during a standardized cooling protocol. Source of illustration: Shutterstock (
, 2018, no. 100687138).
Figure 3
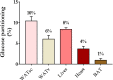
Whole body glucose uptake into brown adipose tissues and muscles during acute cold exposure. During mild cold exposure, glucose uptake is stimulated in brown adipose tissue, but also in several centrally-located skeletal muscles. Brown adipose tissue glucose uptake is ~8-fold higher than that of skeletal muscles, on average, per gram of tissue during mild cold exposure. However, total mass of brown adipose tissue is about 0.2% of that of skeletal muscles. Therefore, brown adipose tissue and skeletal muscle glucose uptake account for ~1 and 50%, respectively, of systemic glucose disposal. The figures presented were calculated from previously published data in young healthy individuals, before cold acclimation (39). BAT, brown adipose tissue; SUV, standard uptake value. Source of muscle illustration: Shutterstock (
, 2018, no. 404668558).
Figure 4
Organ-specific glucose partitioning during acute cold exposure. The figures presented were calculated from a previously published study in young healthy individuals, before cold acclimation (39), based on calculations that we detailed previously (110). BAT, brown adipose tissue; WATsc, sub-cutaneous white adipose tissues; WATv, visceral white adipose tissue.
Figure 5
Brown adipose tissue uptake of energy substrates. Total brown adipose tissue uptake of energy substrates is calculated from published quantitative, dynamic positron emission tomography or microdialysis experiments in humans, multiplied by a typical total brown adipose tissue mass reported in the literature. Data from (73, 108, 113), (108, 113, 114), (115), and (116) were used to calculate glucose, NEFA, dietary fatty acid, and glutamate BAT uptake, respectively. 18FDG, 18-fluorodeoxyglucose; BAT, brown adipose tissue; NEFA, nonesterified fatty acids; PET/CT, positron emission tomography coupled with computed tomography.
Figure 6
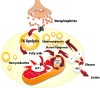
Glucose metabolism in brown adipose tissue. Most of the glucose taken up by brown adipose tissue during cold exposure does not contribute to thermogenesis. Experimental data show that approximately half of the glucose molecules are excreted from brown adipose tissue as lactate. Most of the remaining glucose likely contributes to glycerol production (glyceroneogenesis) and/or fatty acid synthesis (de novo lipogenesis) for intracellular triglyceride synthesis. The mitochondrion illustration was obtained free of copyright from Pixabay (
, 2018).
Figure 7
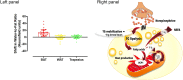
Intracellular triglyceride (TG) mobilization in brown adipose tissue during cold exposure. Left panel: Brown adipose tissue (BAT), white adipose tissue (WAT) and trapezius muscle change in radio-density during standardized acute cold exposure from previously published studies of our group (39, 73, 108, 109). Right panel: During cold-induced brown adipose tissue metabolic activation, up to 8 g of intracellular triglycerides can be mobilized within 2 h. The metabolic fate of the fatty acids that are mobilized is currently unknown. Although these fatty acids likely constitute most of the energy substrates driving brown adipose tissue thermogenesis, a fraction of them may also be released in circulation to be utilized by other tissues. NEFA, nonesterified fatty acids; UCP1, uncoupling protein 1. The mitochondrion illustration was obtained free of copyright from Pixabay (
, 2018).
Figure 8
Brown adipose tissue (BAT) oxidative metabolism and contribution to total body energy expenditure. Brown adipose tissue oxygen consumptions are from U Din et al. (143) and brown adipose tissue-containing adipose tissue (AT) mass range is from Leitner et al. (61). Calculations were made assuming energy expenditure of 4.801 kcal per liter of oxygen consumed (201) and an adipose tissue density of 0.925 g.ml−1 (202). AT, adipose tissue.
Similar articles
- Translational Pharmacology and Physiology of Brown Adipose Tissue in Human Disease and Treatment.
Larson CJ. Larson CJ. Handb Exp Pharmacol. 2019;251:381-424. doi: 10.1007/164_2018_184. Handb Exp Pharmacol. 2019. PMID: 30689089 - Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans.
Ouellet V, Labbé SM, Blondin DP, Phoenix S, Guérin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Ouellet V, et al. J Clin Invest. 2012 Feb;122(2):545-52. doi: 10.1172/JCI60433. Epub 2012 Jan 24. J Clin Invest. 2012. PMID: 22269323 Free PMC article. - 15O PET measurement of blood flow and oxygen consumption in cold-activated human brown fat.
Muzik O, Mangner TJ, Leonard WR, Kumar A, Janisse J, Granneman JG. Muzik O, et al. J Nucl Med. 2013 Apr;54(4):523-31. doi: 10.2967/jnumed.112.111336. Epub 2013 Jan 29. J Nucl Med. 2013. PMID: 23362317 Free PMC article. - Substrate Utilization by Brown Adipose Tissue: What's Hot and What's Not?
McNeill BT, Morton NM, Stimson RH. McNeill BT, et al. Front Endocrinol (Lausanne). 2020 Sep 25;11:571659. doi: 10.3389/fendo.2020.571659. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33101206 Free PMC article. Review. - Near-Infrared Time-Resolved Spectroscopy for Assessing Brown Adipose Tissue Density in Humans: A Review.
Hamaoka T, Nirengi S, Fuse S, Amagasa S, Kime R, Kuroiwa M, Endo T, Sakane N, Matsushita M, Saito M, Yoneshiro T, Kurosawa Y. Hamaoka T, et al. Front Endocrinol (Lausanne). 2020 May 19;11:261. doi: 10.3389/fendo.2020.00261. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 32508746 Free PMC article. Review.
Cited by
- Mirabegron: The most promising adipose tissue beiging agent.
Bel JS, Tai TC, Khaper N, Lees SJ. Bel JS, et al. Physiol Rep. 2021 Mar;9(5):e14779. doi: 10.14814/phy2.14779. Physiol Rep. 2021. PMID: 33650753 Free PMC article. Review. - Emodin Improves Glucose and Lipid Metabolism Disorders in Obese Mice via Activating Brown Adipose Tissue and Inducing Browning of White Adipose Tissue.
Cheng L, Zhang S, Shang F, Ning Y, Huang Z, He R, Sun J, Dong S. Cheng L, et al. Front Endocrinol (Lausanne). 2021 May 10;12:618037. doi: 10.3389/fendo.2021.618037. eCollection 2021. Front Endocrinol (Lausanne). 2021. PMID: 34040579 Free PMC article. - Adipose Tissue Dysfunction Related to Climate Change and Air Pollution: Understanding the Metabolic Consequences.
Stojchevski R, Chandrasekaran P, Hadzi-Petrushev N, Mladenov M, Avtanski D. Stojchevski R, et al. Int J Mol Sci. 2024 Jul 18;25(14):7849. doi: 10.3390/ijms25147849. Int J Mol Sci. 2024. PMID: 39063092 Free PMC article. Review. - Measurement of Futile Creatine Cycling Using Respirometry.
Rahbani JF, Chouchani ET, Spiegelman BM, Kazak L. Rahbani JF, et al. Methods Mol Biol. 2022;2448:141-153. doi: 10.1007/978-1-0716-2087-8_10. Methods Mol Biol. 2022. PMID: 35167096 Free PMC article. - Brown Adipose Tissue-A Translational Perspective.
Carpentier AC, Blondin DP, Haman F, Richard D. Carpentier AC, et al. Endocr Rev. 2023 Mar 4;44(2):143-192. doi: 10.1210/endrev/bnac015. Endocr Rev. 2023. PMID: 35640259 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous