ESCRT-III mediates budding across the inner nuclear membrane and regulates its integrity - PubMed (original) (raw)
ESCRT-III mediates budding across the inner nuclear membrane and regulates its integrity
Jun Arii et al. Nat Commun. 2018.
Abstract
Vesicle-mediated nucleocytoplasmic transport is a nuclear pore-independent mechanism for the nuclear export of macromolecular complexes, but the molecular basis for this transport remains largely unknown. Here we show that endosomal sorting complex required for transport-III (ESCRT-III) is recruited to the inner nuclear membrane (INM) during the nuclear export of herpes simplex virus 1 (HSV-1). Scission during HSV-1 budding through the INM is prevented by depletion of ESCRT-III proteins. Interestingly, in uninfected human cells, the depletion of ESCRT-III proteins induces aberrant INM proliferation. Our results show that HSV-1 expropriates the ESCRT-III machinery in infected cells for scission of the INM to produce vesicles containing progeny virus nucleocapsids. In uninfected cells, ESCRT-III regulates INM integrity by downregulating excess INM.
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
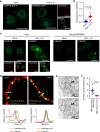
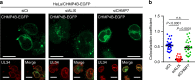
ESCRT-III is recruited to the nuclear rim in HSV-1-infected cells. a HeLa/CHMP4B-EGFP cells mock-infected or infected with HSV-1 for 22 h were analyzed by confocal microscopy for CHMP4B-EGFP and lamin A/C. Bars, 20 μm. Images are representative of three independent experiments. b Colocalization between CHMP4B-EGFP and lamin A/C in the experiment in (a) was quantified using Mander’s colocalization coefficient. Data are shown as the mean ± SEM (n = 16 for mock-infected and 15 for HSV-1-infected cells representative of three independent experiments). c HeLa or HeLa/CHMP4B KO cells were mock-infected or infected with HSV-1 for 22 h and analyzed by confocal microscopy for CHMP4B and lamin A/C. Bars, 20 μm. Images are representative of three independent experiments. d HeLa/CHMP4B-EGFP cells infected with HSV-1 for 22 h were analyzed by N-SIM super-resolution microscopy for (left) CHMP4B-EGFP and calnexin or (right) CHMP4B-EGFP and lamin A/C. Bars, 1 μm. Fluorescence line scans along the dotted lines of N-SIM images are shown under each image. Images are representative of three independent experiments. e HeLa/CHMP4B-EGFP cells were infected with HSV-1 for 24 h and analyzed by immunoelectron microscopy. C cytoplasm, N nucleus, NM, nuclear membrane. Bars, 200 nm. Arrowheads indicate localization of CHMP4P-EGFP labeled with anti-GFP antibody along the INM and in primary enveloped virions. In the lower panel, straight lines indicate the INM and dotted lines indicate the ONM and the envelope of virions in the perinuclear space. Images are representative of three independent experiments. f Quantification of gold particles on the NM with or without virions in the experiment in (e). Seven areas of each section were analyzed and the data are shown as the mean ± SEM. Data are representative of two independent experiments. The indicated _P_-values were obtained using the unpaired Student’s _t_-test (b, f)
Fig. 2
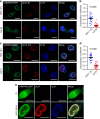
HSV-1 recruits ESCRT-III via the viral NEC. a Confocal microscope images of HeLa/CHMP4B-EGFP cells infected with wild-type HSV-1 or HSV-1 ΔUL34. Bars, 20 μm. Images are representative of three independent experiments. b Colocalization between CHMP4B-EGFP and lamin A/C in the experiment in (a) was quantified using Mander’s colocalization coefficient. Data are shown as the mean ± SEM (n = 21 for wild-type HSV-1 or 26 for HSV-1 ΔUL34-infected cells and are representative of three independent experiments). c Confocal microscope images of HeLa/CHMP4B-EGFP cells infected with wild-type HSV-1 or HSV-1 ΔUL31. Bars, 20 μm. Images are representative of three independent experiments. d Colocalization between CHMP4B-EGFP and lamin A/C was quantified using Mander’s colocalization coefficient in the experiment in (c). Data are shown as the mean ± SEM (n = 20 for wild-type HSV-1 or 21 for HSV-1 ΔUL31-infected cells in representative of three independent experiments). e Confocal microscope images of HeLa/CHMP4B-EGFP cells transfected with the UL31 and UL34 expression vectors. Bars, 10 μm. Images are representative of three independent experiments. The indicated _P_-values were obtained using the unpaired Student’s _t_-test (b, d)
Fig. 3
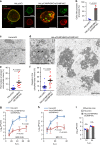
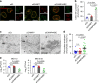
ESCRT-III is required for proper nuclear egress of HSV-1. a Confocal microscope images of HeLa and HeLa/CHMP4B KO cells treated with (left) control siRNA (siCt) or (Right) siRNAs to CHMP4A and CHMP4C (siCHMP4AC), respectively, for 48 h, and subsequently infected with HSV-1 for 22 h. Bars, 20 μm. Images are representative of three independent experiments. b Percent of cells (80–200 cells in each experiment) with aberrant punctate structures along with the nuclear rim in the experiment in (a). Data are shown as the mean ± SEM of three independent experiments. Electron microscope images of (c) HeLa and d HeLa/CHMP4B KO cells treated with siCt or siCHMP4AC, respectively, for 48 h and infected with HSV-1 for 22 h. Arrowheads indicate virions defective in the scission steps. C cytoplasm, N nucleus, NM nuclear membrane. Bar, 500 nm. Images are representative of three independent experiments. Percent of (e) perinuclear enveloped virions and f capsids in the cytoplasm of 14 cells in the experiments in (c, d). Data are shown as the mean ± SEM and are representative of three independent experiments. g–i HeLa and HeLa/CHMP4B KO cells treated with siRNA(s) as described in a were infected with HSV-1 at an MOI of (g) 10 or h 0.05, or with i influenza virus at an MOI of 0.01, and progeny virus titers were assayed at the indicated hours post infection (h.p.i). Data are shown as the mean ± SEM of three independent experiments. The indicated _P_-values were obtained using the unpaired Student’s _t_-test (b, e–i). n.s., not significant
Fig. 4
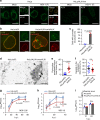
ESCRT-III adaptor protein ALIX contributes to HSV-1 nuclear egress. a Confocal microscope images of HeLa or HeLa/ALIX-low cells mock-infected or infected with HSV-1 for 22 h and stained with anti-ALIX and anti-UL34 antibodies. Bars, 20 μm. Images are representative of three independent experiments. b Confocal microscope images of HeLa and HeLa/ALIX-low cells treated with control siRNA (siCt) or siRNA to ALIX (siALIX), respectively, for 48 h, and then infected with HSV-1 for 22 h. Bars, 20 μm. Images are representative of three independent experiments. c Percent of cells (50–100 cells in each experiment) with aberrant punctate structures along with the nuclear rim in the experiment in (b). Data are shown as the mean ± SEM of three independent experiments. d Electron microscope images of HeLa and HeLa/ALIX-low cells treated with siCt or siALIX, respectively, for 48 h and infected with HSV-1 for 22 h. C cytoplasm, N nucleus, NM nuclear membrane. Bars, 500 nm. Arrowheads indicate virions defective in the scission steps. Images are representative of three independent experiments. Percent of (e) perinuclear enveloped virions and f capsids in the cytoplasm of 13 cells in the experiment in (d) was determined. Data are shown as the mean ± SEM and are representative of three independent experiments. g–i HeLa and HeLa/ALIX-low cells treated with siRNA as described in (b) were infected with HSV-1 at an MOI of (g) 0.05 or (h) 10, or with i influenza virus at an MOI of 0.01, and progeny virus titers were assayed at the indicated hours post infection (h.p.i.). Data are shown as the mean ± SEM of (g, i) three or (h) four independent experiments. The indicated _P_-values were obtained using the unpaired Student’s _t_-test (c, e–i). n.s., not significant
Fig. 5
ALIX is required for the recruitment of ESCRT-III to the NM in HSV-1-infected cells. a Confocal microscope images of HeLa/CHMP4B-EGFP cells treated with (left) control siRNA (siCt), (middle) siRNA to ALIX (siALIX), or (right) siRNA to CHMP7 (siCHMP7) for 48 h and subsequently infected with HSV-1 for 22 h. Bars, 20 μm. Images are representative of three independent experiments. b Colocalization between CHMP4B-EGFP and UL34 in the experiment in (a) was quantified using Mander’s colocalization coefficient. Data are shown as the mean ± SEM (n = 26 for siCt, 29 for siALIX and 23 for siCHMP7, and are representative of three independent experiments). The indicated _P_-values were obtained using the Tukey’s test. n.s., not significant
Fig. 6
Effects of CHMP7 depletion on HSV-1 replication. a Confocal microscope images of HeLa cells treated with control siRNA (siCt), siRNA to CHMP7 (siCHMP7), or siRNA to CHMP4A, B, and C (siCHMP4ABC) for 48 h and infected with HSV-1 for 22 h. Images are representative of three independent experiments. Bars, 20 μm. b The percent of cells (100–200 cells in each experiment) with aberrant punctate structures along with the nuclear rim was determined in the experiment in (a). Data are shown as the mean ± SEM of three independent experiments. c Electron microscope images of HeLa cells treated with siRNA as described in a and infected with HSV-1 for 22 h. C cytoplasm, N nucleus, NM nuclear membrane. Bars, 500 nm. Images are representative of three independent experiments. d The percent of perinuclear enveloped virions in 15 cells in the experiment in (c) was determined. Data are shown as the mean ± SEM and are representative of three independent experiments. e HeLa cells treated with siRNA as described in (a) were infected with HSV-1 at an MOI of 10 or 0.05 and viral titers were assayed at the indicated times post infection. Data are shown as the mean ± SEM of five (MOI 10) or six independent experiments (MOI 0.05). The indicated _P_-values were obtained using the Tukey’s test (b, d, e). n.s., not significant
Fig. 7
Ectopic expression of the dominant-negative mutant of VPS4 inhibits HSV-1 nuclear egress. a Electron microscope images of HeLa cells co-infected with HSV-1 and either control recombinant adenovirus (Ad-Ct) or recombinant adenovirus expressing Flag-VPS4-DN (Ad-VPS4-DN). Cells were infected with Ad-Ct or Ad-VPS4-DN for 4 h and then co-infected with HSV-1 for 22 h. Arrowheads indicate virions defective in scission steps in the aberrant invagination structures derived from the INM. C cytoplasm, N nucleus, NM nuclear membrane. Bars, 500 nm. Images are representative of three independent experiments. The percent of (b) perinuclear enveloped virions and c capsids in the cytoplasm of the cells in the experiment in (a) were determined. Data are shown as the mean ± SEM for 25 cells and are representative of three independent experiments. The indicated _P_-values were obtained using the Tukey’s test (b, c)
Fig. 8
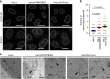
ESCRT-III is required for efficient vesicle-mediated nucleocytoplasmic transport in Drosophila S2 cells. a Confocal microscope images of S2 cells treated with control dsRNA (dsRNA-Ct) or dsRNA to shrub (dsRNA-shrub), the Drosophila ortholog of mammalian CHMP4. Arrowheads indicate the Fz2 foci associated with the nucleus. Bars, 5 μm. Images are representative of three independent experiments. b The Fz2-positive punctate structures in dsRNA-treated S2 cells in the experiment in (a) were quantified. Data are shown as the mean ± SEM for 100 cells and are representative of three independent experiments. c Electron microscope images of S2 cells treated with dsRNA-Ct or dsRNA-shrub. Arrowheads indicate the INM invagination structures with large electron-dense granules. C cytoplasm, N nucleus, NM nuclear membrane. Bars, 500 nm. Images are representative of three independent experiments. d The invagination structures with electron-dense granules in dsRNA-treated S2 cells in the experiment in (c) were quantified. Data are shown as the mean ± SEM for 101 cells and are representative of 3 independent experiments. The indicated _P_-values were obtained using the Tukey’s test (b, d)
Fig. 9
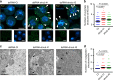
ESCRT-III contributes to maintenance of the integrity of the inner nuclear membrane in normal HeLa cells. a Confocal microscope images of HeLa, HeLa/CHMP4B KO, and HeLa/ALIX-low cells stained with Lamin A/C (top row) or Emerin (bottom row). Bars, 20 μm. Images are representative of 3 independent experiments. b The number of trans-nuclear tubes in the experiments in (a) was measured (n = 74 for HeLa, n = 55 or HeLa/CHMP4B KO, n = 63 for HeLa/ALIX-low). Data are shown as the mean ± SEM and are representative of three independent experiments. The indicated _P_-values were obtained using the Tukey’s test. c Electron microscope images of the cells in (a). Arrowheads indicate trans-nuclear tubes derived from the INM. C cytoplasm, N nucleus, NM nuclear membrane. Bars, 500 nm. Images are representative of three independent experiments
Fig. 10
Model for INM scission by ESCRT-III. a In HSV-1-infected cells, ESCRT-III is recruited to INM sites, where HSV-1 capsids acquire a primary envelope that functions in INM scission to produce primary enveloped virions in the perinuclear space. Depletion of CHMP4 proteins impairs primary envelopment and produces an accumulation of primary enveloped virions in the invagination structures in the nucleus. Nuclear morphology is maintained by the lamina meshwork but HSV-1 infection dissociates nuclear lamina. Thus, arrested virions might be mainly accumulated in the invagination structures derived from the INM. In normal (uninfected) human cells, ESCRT-III contributes to downregulate excess INM. This process might be similar to the vesicle-mediated nucleocytoplasmic transport of HSV-1 nucleocapsids. CHMP4 KO increases the INM proliferation in a manner independent of cell cycle. b Proposed model of vesicle-mediated nucleocytoplasmic transport of HSV-1 nucleocapsids. (i) Protein kinases recruited by the NEC induce local dissolution of the nuclear lamina to allow nucleocapsids access to the INM. Host protein p32 contributes to the recruitment of protein kinase C. (ii) The NEC deforms the INM to wrap around the nucleocapsid. (iii) The NEC recruits ESCRT-III machinery via ALIX and mediates INM scission to complete primary envelopment. (iv) The de-envelopment process is still unclear, but a possible role of viral gB and gH together with host protein CD98hc, β1 integrin, and p32 has been reported,,. Phosphorylation of the NEC by the viral Us3 protein kinase promotes de-envelopment. The torsin/LULL1 complex may indirectly contribute to this step,
Similar articles
- Role of the Arginine Cluster in the Disordered Domain of Herpes Simplex Virus 1 UL34 for the Recruitment of ESCRT-III for Viral Primary Envelopment.
Arii J, Takeshima K, Maruzuru Y, Koyanagi N, Nakayama Y, Kato A, Mori Y, Kawaguchi Y. Arii J, et al. J Virol. 2022 Jan 26;96(2):e0170421. doi: 10.1128/JVI.01704-21. Epub 2021 Nov 3. J Virol. 2022. PMID: 34730397 Free PMC article. - Herpes Simplex Virus 1 UL34 Protein Regulates the Global Architecture of the Endoplasmic Reticulum in Infected Cells.
Maeda F, Arii J, Hirohata Y, Maruzuru Y, Koyanagi N, Kato A, Kawaguchi Y. Maeda F, et al. J Virol. 2017 May 26;91(12):e00271-17. doi: 10.1128/JVI.00271-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28356536 Free PMC article. - Herpes Simplex Virus 1 Recruits CD98 Heavy Chain and β1 Integrin to the Nuclear Membrane for Viral De-Envelopment.
Hirohata Y, Arii J, Liu Z, Shindo K, Oyama M, Kozuka-Hata H, Sagara H, Kato A, Kawaguchi Y. Hirohata Y, et al. J Virol. 2015 Aug;89(15):7799-812. doi: 10.1128/JVI.00741-15. Epub 2015 May 20. J Virol. 2015. PMID: 25995262 Free PMC article. - [Cellular ESCRT complex and its roles in enveloped viruses budding].
Li Z, Tian H, Liu T. Li Z, et al. Sheng Wu Gong Cheng Xue Bao. 2012 Sep;28(9):1031-7. Sheng Wu Gong Cheng Xue Bao. 2012. PMID: 23289305 Review. Chinese. - Border Safety: Quality Control at the Nuclear Envelope.
Webster BM, Lusk CP. Webster BM, et al. Trends Cell Biol. 2016 Jan;26(1):29-39. doi: 10.1016/j.tcb.2015.08.002. Epub 2015 Oct 1. Trends Cell Biol. 2016. PMID: 26437591 Free PMC article. Review.
Cited by
- Viral use and subversion of membrane organization and trafficking.
Hernandez-Gonzalez M, Larocque G, Way M. Hernandez-Gonzalez M, et al. J Cell Sci. 2021 Mar 4;134(5):jcs252676. doi: 10.1242/jcs.252676. J Cell Sci. 2021. PMID: 33664154 Free PMC article. Review. - Role of Phosphatidylethanolamine Biosynthesis in Herpes Simplex Virus 1-Infected Cells in Progeny Virus Morphogenesis in the Cytoplasm and in Viral Pathogenicity In Vivo.
Arii J, Fukui A, Shimanaka Y, Kono N, Arai H, Maruzuru Y, Koyanagi N, Kato A, Mori Y, Kawaguchi Y. Arii J, et al. J Virol. 2020 Nov 23;94(24):e01572-20. doi: 10.1128/JVI.01572-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32999028 Free PMC article. - The human cytomegalovirus decathlon: Ten critical replication events provide opportunities for restriction.
Turner DL, Mathias RA. Turner DL, et al. Front Cell Dev Biol. 2022 Nov 25;10:1053139. doi: 10.3389/fcell.2022.1053139. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36506089 Free PMC article. Review. - An ESCRT/VPS4 Envelopment Trap To Examine the Mechanism of Alphaherpesvirus Assembly and Transport in Neurons.
Barnes J, Jordan BA, Wilson DW. Barnes J, et al. J Virol. 2022 Mar 23;96(6):e0217821. doi: 10.1128/jvi.02178-21. Epub 2022 Jan 19. J Virol. 2022. PMID: 35045266 Free PMC article. - Molecular Mechanisms for the Regulation of Nuclear Membrane Integrity.
Lee GE, Byun J, Lee CJ, Cho YY. Lee GE, et al. Int J Mol Sci. 2023 Oct 23;24(20):15497. doi: 10.3390/ijms242015497. Int J Mol Sci. 2023. PMID: 37895175 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases