β-Defensins Coordinate In Vivo to Inhibit Bacterial Infections of the Trachea - PubMed (original) (raw)
β-Defensins Coordinate In Vivo to Inhibit Bacterial Infections of the Trachea
Lisa Kathleen Ryan et al. Vaccines (Basel). 2018.
Abstract
β-defensins are predicted to play an important role in innate immunity against bacterial infections in the airway. We previously observed that a type III-secretion product of Bordetella bronchiseptica inhibits the NF-κB-mediated induction of a β-defensin in airway epithelial cells in vitro. To confirm this in vivo and to examine the relative roles of other β-defensins in the airway, we infected wild-type C57BL/6 mice and mice with a deletion of the mBD-1 gene with B. bronchiseptica wild-type strain, RB50 and its mutant strain lacking the type III-secretion system, WD3. The bacteria were quantified in the trachea and the nasal tissue and mRNA levels of mouse β-defensin-3 (mBD-3) were assessed after 24 h. Infection with the wild-type bacterial strain resulted in lower mBD-3 mRNA levels in the trachea than in mice infected with the type III-deficient strain. Furthermore, we observed an increase in bacterial numbers of RB50 only in the tracheas of mBD-1-deficient mice. Neutrophils were also more abundant on the trachea in RB50 infected WT mice but not in the bronchiolar lavage fluid (BAL), compared with WD3 infected WT and mBD-1-/- mice, indicating that the coordination of β-defensin chemotactic effects may be confined to tracheal epithelial cells (TEC). RB50 decreased the ability of mice to mount an early specific antibody response, seven days after infection in both WT and mBD-1-/- mice but there were no differences in titers between RB50-infected WT and mBD-1-/- mice or between WD3-infected WT and mBD-1-/- mice, indicating mBD-1 was not involved in induction of the humoral immune response to the B. bronchiseptica. Challenge of primary mouse TEC in vitro with RB50 and WD3, along with IL-1β, further corroborated the in vivo studies. The results demonstrate that at least two β-defensins can coordinate early in an infection to limit the growth of bacteria in the trachea.
Keywords: Bordetella bronchiseptica; epithelial cells; innate immunity; β-defensins.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript and in the decision to publish the results.
Figures
Figure 1
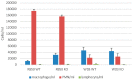
Colonization with Bordetella bronchiseptica wild type strain (RB50) in the trachea and nose in C57Bl/6 wild type mice (WT) during the course of infection. Mice were intranasally inoculated with varying cfu (600, 3000 and 10,000) in 50 µL PBS and tracheas and nasal epithelium were excised after 3, 7 and 20 days. Tissues were homogenized, cultured and cfu/mg trachea (A) and cfu/mg nasal tissue (B) were determined. Increasing inoculums led to increased cfu with RB50. Colonization was evident at 3 days, peaked at 7 days and was nearly gone by 20 days with these doses.
Figure 2
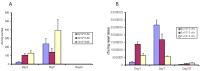
In vivo inhibition of β-defensin expression by the type III secretion system of B. bronchiseptica. (A) Wild-type mice (C57Bl/6) were intranasally inoculated with 1 × 104 cfu wild-type bacteria (RB50) or its type III secretion deficient (WD3) strain in 50 µL (or 50 µL PBS). After 24 h, 3 days and 7 days, tracheas were excised, mRNA was extracted and relative mBD-3 levels were quantified by qRT-PCR, normalized to β-actin. Line or asterisk indicates a significant increase in mBD-3 mRNA after 24 h and at 3 days (p ≤ 0.05). (B) Wild-type mice (C57Bl/6) were intranasally inoculated with 1 × 105 cfu wild-type bacteria (RB50) or its type III secretion deficient (WD3) strain in 50 µL (or 50 µL PBS). After 24 h, tracheas were excised, mRNA was extracted and relative mBD-3, mBD-14 and KC levels were quantified by qRT-PCR. Asterisk denotes a significant difference between RB50 and WD3 infections as determined by Student’s _t_-test (p ≤ 0.05). Error bars = SEM.
Figure 3
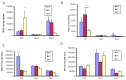
In vivo coordination of β-defensin activity to inhibit bacterial colonization in the trachea but not the nose in the first 24 h. 105 cfu of B. bronchiseptica RB50 or WD3 were intranasally inoculated into WT and mBD-1-deficient mice. After 24 h, tracheas and nasal tissue were excised, homogenized and plated on selective media to enumerate B. bronchiseptica colonies. (A) RB50, trachea; (B) WD3, trachea; (C) RB50, nose; (D) WD3, nose. * denotes significant increase in mBD1−/− tracheas vs. WT and HT and in HT vs. KO (p ≤ 0.03); ** significant increase in WT nasal tissue vs. HT and KO (p < 0.0002). Error bars = SEM.
Figure 4
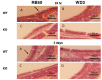
Neutrophil influx in bacteria-infected tracheas in wild-type (WT) and in mBD-1-deficient mice. 105 cfu B. bronchiseptica strain RB50 (A,B) or WD3 (C,D) were intranasally inoculated into WT (A,C) or mBD-1-deficient (B,D) mice. After 24 h, tracheas were excised, fixed and processed for histology. Tissue sections were stained with H&E and examined by light microscopy. Neutrophils were most visible on the surface of tracheas of wild-type mice infected with RB50, with the greatest number occurring at Day 3 after infection. Magnification = 400×.
Figure 5
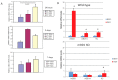
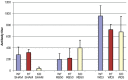
Neutrophil influx into the airways of infected mice. Wild type C57Bl/6 mice or mBD-1(−/−) mice were infected with 1 × 105 RB50 wild type bacteria or WD3 mutant bacteria as indicated. Mice were lavaged with HBSS-24 h following infection. Bronchoalveolar lavage (BAL) cells were stained with trypan blue dye and viable cells counted. Viability was >95%. Cell morphology was determined on cytospun BAL cells via Diff-Quik stain. Alveolar macrophages are indicated by blue bars, neutrophils by red bars and lymphocytes by green bars. Sham-infected mice had 1 × 105 total cells/mL BAL, with 98% alveolar macrophages and did not differ between WT and mBD-1(−/−) mice.
Figure 6
Early serum antibody titers in mice infected with RB50 or WD3 strains of B. bronchiseptica seven days after infection (ELISA). Blood was collected from WT, HT and mBD-1−/− mice infected with 1 × 104 RB50 or WD3 for serum IgM, IgG and IgA combined antibody titers against either strain of B. bronchiseptica using a secondary antibody that detects all three Ig subtypes. Blood was collected on days 3, 7 and 20 following infection. Black bars indicate the denominator of mean antibody titer in WT mice, blue bars indicate that of HT mice and red bars indicate that of mBD-1−/− mice. Error bars indicate SEM (n = 5). Infection was the same infection as Figure 1.
Figure 7
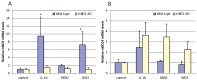
In vitro challenge of primary mouse tracheal epithelial cells from WT and mBD-1−/− mice with RB50, WD3 and IL-1β. Tracheas were excised from either WT or mBD-1−/− mice and grown on airway-liquid interface cultures, allowing differentiation of the tracheal epithelial cells (TEC). 100 ng/mL IL-1β, or 1,000:1 MOI of RB50 or WD3 was added to the apical surface for 6 h. Total mRNA was isolated from the TEC and levels of mBD-3 (A) and mBD-14 (B) mRNA were quantified by qRT-PCR. n = 3 cultures; error bars = +/− SEM. Statistical comparison was done using the Student’s _t_-test with significance (*) at p ≤ 0.05.
Similar articles
- Suppression of NF-kappaB-mediated beta-defensin gene expression in the mammalian airway by the Bordetella type III secretion system.
Legarda D, Klein-Patel ME, Yim S, Yuk MH, Diamond G. Legarda D, et al. Cell Microbiol. 2005 Apr;7(4):489-97. doi: 10.1111/j.1462-5822.2004.00473.x. Cell Microbiol. 2005. PMID: 15760449 Free PMC article. - Enhanced expression of murine beta-defensins (MBD-1, -2,- 3, and -4) in upper and lower airway mucosa of influenza virus infected mice.
Chong KT, Thangavel RR, Tang X. Chong KT, et al. Virology. 2008 Oct 10;380(1):136-43. doi: 10.1016/j.virol.2008.07.024. Epub 2008 Aug 27. Virology. 2008. PMID: 18752820 - Role of Bordetella bronchiseptica fimbriae in tracheal colonization and development of a humoral immune response.
Mattoo S, Miller JF, Cotter PA. Mattoo S, et al. Infect Immun. 2000 Apr;68(4):2024-33. doi: 10.1128/IAI.68.4.2024-2033.2000. Infect Immun. 2000. PMID: 10722598 Free PMC article. - Interleukin-1 receptor-1-deficient mice show attenuated production of ocular surface inflammatory cytokines in experimental dry eye.
Narayanan S, Corrales RM, Farley W, McDermott AM, Pflugfelder SC. Narayanan S, et al. Cornea. 2008 Aug;27(7):811-7. doi: 10.1097/ICO.0b013e31816bf46c. Cornea. 2008. PMID: 18650668 - A novel murine beta -defensin expressed in tongue, esophagus, and trachea.
Jia HP, Wowk SA, Schutte BC, Lee SK, Vivado A, Tack BF, Bevins CL, McCray PB Jr. Jia HP, et al. J Biol Chem. 2000 Oct 27;275(43):33314-20. doi: 10.1074/jbc.M006603200. J Biol Chem. 2000. PMID: 10922379
Cited by
- Bovine Neutrophil β-Defensin-5 Provides Protection against Multidrug-Resistant Klebsiella pneumoniae via Regulating Pulmonary Inflammatory Response and Metabolic Response.
Zhu S, Dai D, Li H, Huang J, Kang W, Yang Y, Zhong Y, Xiang Y, Liu C, He J, Liang Z. Zhu S, et al. Int J Mol Sci. 2024 Sep 29;25(19):10506. doi: 10.3390/ijms251910506. Int J Mol Sci. 2024. PMID: 39408834 Free PMC article. - Immunomodulatory Peptides as Vaccine Adjuvants and Antimicrobial Agents.
Hemmati S, Saeidikia Z, Seradj H, Mohagheghzadeh A. Hemmati S, et al. Pharmaceuticals (Basel). 2024 Feb 2;17(2):201. doi: 10.3390/ph17020201. Pharmaceuticals (Basel). 2024. PMID: 38399416 Free PMC article. - Intranasal B5 promotes mucosal defence against Actinobacillus pleuropneumoniae via ameliorating early immunosuppression.
Huang J, Kang W, Yi D, Zhu S, Xiang Y, Liu C, Li H, Dai D, Su J, He J, Liang Z. Huang J, et al. Virulence. 2024 Dec;15(1):2316459. doi: 10.1080/21505594.2024.2316459. Epub 2024 Feb 20. Virulence. 2024. PMID: 38378464 Free PMC article. - Promising role of defensins peptides as therapeutics to combat against viral infection.
Solanki SS, Singh P, Kashyap P, Sansi MS, Ali SA. Solanki SS, et al. Microb Pathog. 2021 Jun;155:104930. doi: 10.1016/j.micpath.2021.104930. Epub 2021 Apr 29. Microb Pathog. 2021. PMID: 33933603 Free PMC article. Review. - Immunoregulatory and Antimicrobial Activity of Bovine Neutrophil β-Defensin-5-Loaded PLGA Nanoparticles against Mycobacterium bovis.
Liang Z, Liu Y, Sun X, Lin J, Yao J, Song Y, Li M, Liu T, Zhou X. Liang Z, et al. Pharmaceutics. 2020 Dec 1;12(12):1172. doi: 10.3390/pharmaceutics12121172. Pharmaceutics. 2020. PMID: 33271900 Free PMC article.
References
Grants and funding
- R01DE14897, R01HL67871, AI26806S1, R21AI072245, R03ES016851/National Institutes of Health
- R03 ES016851/ES/NIEHS NIH HHS/United States
- R21 AI072245/AI/NIAID NIH HHS/United States
- R01 DE014897/DE/NIDCR NIH HHS/United States
- R01 HL067871/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials