Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers - PubMed (original) (raw)
Review
Non-viral Delivery of Nucleic Acids: Insight Into Mechanisms of Overcoming Intracellular Barriers
Mikhail Durymanov et al. Front Pharmacol. 2018.
Abstract
Delivery of genes, including plasmid DNAs, short interfering RNAs (siRNAs), and messenger RNAs (mRNAs), using artificial non-viral nanotherapeutics is a promising approach in cancer gene therapy. However, multiple physiological barriers upon systemic administration remain a key challenge in clinical translation of anti-cancer gene therapeutics. Besides extracellular barriers including sequestration of gene delivery nanoparticles from the bloodstream by resident organ-specific macrophages, and their poor extravasation and tissue penetration in tumors, overcoming intracellular barriers is also necessary for successful delivery of nucleic acids. Whereas for RNA delivery the endosomal barrier holds a key importance, transfer of DNA cargo additionally requires translocation into the nucleus. Better understanding of crossing membrane barriers by nucleic acid nanoformulations is essential to the improvement of current non-viral carriers. This review aims to summarize relevant literature on intracellular trafficking of non-viral nanoparticles and determine key factors toward surmounting intracellular barriers. Moreover, recent data allowed us to propose new interpretations of current hypotheses of endosomal escape mechanisms of nucleic acid nanoformulations.
Keywords: endosomal escape; gene delivery; intracellular trafficking; lipoplexes; polyplexes; siRNA delivery.
Figures
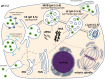
FIGURE 1
Intracellular trafficking of non-viral nucleic acid nanoformulations. Comments can be found in the text. EE, early endosome; MVB, multivesicular body; LE, late endosome; L, lysosome; ERC, endocytic recycling compartment; TGN, _trans_-Golgi network; EEA1, early endosome antigen 1; PI3P, phosphatidylinositol 3-phosphate; PI(3,5)P2, phosphatidylinositol (3,5)-_bis_phosphate; LBPA, lyso_bis_phosphatidic acid; LAMP1, lysosomal-associated membrane protein 1. “+” and “±” mean high and moderate levels of abundance, respectively.
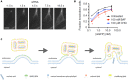
FIGURE 2
Lipoplex endosomal escape. Escape of lipoplex-formulated genetic cargo from an endosome in HeLa cells takes seconds followed by diffusion of siRNA throughout entire cytosol (A). Efficacy of GFP knockdown in HeLa-GFP cells upon incubation with lipoplexes carrying siGFP in the presence of inhibitors of endosomal acidification bafilomycin A1 (BAF) or chloroquine (CHQ) (B). “Lipid-mixing” mechanism of endosomal escape of lipoplex-formulated nucleic acids in assumption of primary role of BMP/LBPA, concentrated in the luminal leaflet of endosomal membrane in area of ILV formation (see the text for additional comments) (C). (A,B) Figures are reprinted by permission from Nature Publishing Group (Wittrup et al., 2015).
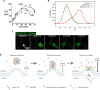
FIGURE 3
Polyplex endosomal escape. Accumulation of chloride in endosomal vesicles upon cellular uptake of polyamidoamine (PAM), polyethylenimine (PEI), and polylysine (POL) reflects higher buffering capacity of PAM and PEI (A). Lack of acidic endosomal pH increase in vesicles containing branched PEI (BPEI), compared to bafilomycin A-treated cells, contradicts the “proton sponge” hypothesis (B). Released polyplex-formulated genetic cargo (FITC-labeled oligodeoxyribonucleotides, ODNs) from an endosome in HeLa cells spreads in a single direction (white arrow) (C). Proposed mechanism of endosomal escape of polyplex-formulated nucleic acids driven by osmotic pressure and local permeabilization of endosomal membrane due to electrostatic interactions between polycation and anionic phospholipids like BMP/LBPA (D). Figure adapted with permission from: (A) (Sonawane et al., 2003), ASBMB; (B) (Benjaminsen et al., 2013), Elsevier; (C) (ur Rehman et al., 2013), copyright (2013) American Chemical Society.
Similar articles
- Modulating intracellular pathways to improve non-viral delivery of RNA therapeutics.
Van de Vyver T, De Smedt SC, Raemdonck K. Van de Vyver T, et al. Adv Drug Deliv Rev. 2022 Feb;181:114041. doi: 10.1016/j.addr.2021.114041. Epub 2021 Nov 8. Adv Drug Deliv Rev. 2022. PMID: 34763002 Review. - Super-resolution Imaging of Proton Sponge-Triggered Rupture of Endosomes and Cytosolic Release of Small Interfering RNA.
Wojnilowicz M, Glab A, Bertucci A, Caruso F, Cavalieri F. Wojnilowicz M, et al. ACS Nano. 2019 Jan 22;13(1):187-202. doi: 10.1021/acsnano.8b05151. Epub 2019 Jan 2. ACS Nano. 2019. PMID: 30566836 - Responsive Nanocarriers as an Emerging Platform for Cascaded Delivery of Nucleic Acids to Cancer.
Liu Y, Xu CF, Iqbal S, Yang XZ, Wang J. Liu Y, et al. Adv Drug Deliv Rev. 2017 Jun 1;115:98-114. doi: 10.1016/j.addr.2017.03.004. Epub 2017 Apr 7. Adv Drug Deliv Rev. 2017. PMID: 28396204 Review. - Mechanism of polyplex- and lipoplex-mediated delivery of nucleic acids: real-time visualization of transient membrane destabilization without endosomal lysis.
ur Rehman Z, Hoekstra D, Zuhorn IS. ur Rehman Z, et al. ACS Nano. 2013 May 28;7(5):3767-77. doi: 10.1021/nn3049494. Epub 2013 Apr 24. ACS Nano. 2013. PMID: 23597090 - Non-viral nucleic acid containing nanoparticles as cancer therapeutics.
Kozielski KL, Rui Y, Green JJ. Kozielski KL, et al. Expert Opin Drug Deliv. 2016 Oct;13(10):1475-87. doi: 10.1080/17425247.2016.1190707. Epub 2016 Jun 6. Expert Opin Drug Deliv. 2016. PMID: 27248202 Free PMC article. Review.
Cited by
- Lipophilic Conjugates for Carrier-Free Delivery of RNA Importable into Human Mitochondria.
Dovydenko I, Meschaninova M, Heckel AM, Tarassov I, Venyaminova A, Entelis N. Dovydenko I, et al. Methods Mol Biol. 2021;2277:49-67. doi: 10.1007/978-1-0716-1270-5_4. Methods Mol Biol. 2021. PMID: 34080144 - Nanoparticles-Delivered Circular RNA Strategy as a Novel Antitumor Approach.
Racca L, Liuzzi E, Comparato S, Giordano G, Pignochino Y. Racca L, et al. Int J Mol Sci. 2024 Aug 16;25(16):8934. doi: 10.3390/ijms25168934. Int J Mol Sci. 2024. PMID: 39201617 Free PMC article. Review. - Optimizing Biocompatibility and Gene Delivery with DMAEA and DMAEAm: A Niacin-Derived Copolymer Approach.
Mapfumo PP, Reichel LS, André T, Hoeppener S, Rudolph LK, Traeger A. Mapfumo PP, et al. Biomacromolecules. 2024 Aug 12;25(8):4749-4761. doi: 10.1021/acs.biomac.4c00007. Epub 2024 Jul 4. Biomacromolecules. 2024. PMID: 38963401 Free PMC article. - Revisiting the Cytotoxicity of Cationic Polyelectrolytes as a Principal Component in Layer-by-Layer Assembly Fabrication.
Naumenko E, Akhatova F, Rozhina E, Fakhrullin R. Naumenko E, et al. Pharmaceutics. 2021 Aug 9;13(8):1230. doi: 10.3390/pharmaceutics13081230. Pharmaceutics. 2021. PMID: 34452190 Free PMC article. - Nucleic acid strategies for infectious disease treatments: The nanoparticle-based oral delivery route.
Chen F, Liu Q, Xiong Y, Xu L. Chen F, et al. Front Pharmacol. 2022 Aug 29;13:984981. doi: 10.3389/fphar.2022.984981. eCollection 2022. Front Pharmacol. 2022. PMID: 36105233 Free PMC article. Review.
References
- Becker M. L., Fagan J. A., Gallant N. D., Bauer B. J., Bajpai V., Hobbie E. K., et al. (2007). Length-dependent uptake of DNA-wrapped single-walled carbon nanotubes. Adv. Mater. 19 939–945. 10.1002/adma.200602667 - DOI
- Bishop C. J., Majewski R. L., Guiriba T.-R. M., Wilson D. R., Bhise N. S., Quiñones-Hinojosa A., et al. (2016). Quantification of cellular and nuclear uptake rates of polymeric gene delivery nanoparticles and DNA plasmids via flow cytometry. Acta Biomater. 37 120–130. 10.1016/j.actbio.2016.03.036 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources