Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses - PubMed (original) (raw)
Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses
Ye Wang et al. Viruses. 2018.
Abstract
Ginseng products used as herb nutritional supplements are orally consumed and fermented to ginsenoside compounds by the intestinal microbes. In this study, we investigated antiviral protective effects of fermented ginseng extracts against different strains of influenza viruses in genetically diverse mouse models. Intranasal coinoculation of mice with fermented ginseng extract and influenza virus improved survival rates and conferred protection against H1N1, H3N2, H5N1, and H7N9 strains, with the efficacy dependent on the dose of ginseng samples. Antiviral protection by fermented ginseng extract was observed in different genetic backgrounds of mice and in the deficient conditions of key adaptive immune components (CD4, CD8, B cell, MHCII). The mice that survived primary virus inoculation with fermented ginseng extract developed immunity against the secondary infection with homologous and heterosubtypic viruses. In vitro cell culture experiments showed moderate virus neutralizing activity by fermented ginseng extract, probably by inhibiting hemagglutination and neuraminidase activity. This study suggests that fermented ginseng extracts might provide a means to treat influenza disease regardless of virus strains.
Keywords: antiviral activity; fermented ginseng; hemagglutinin; influenza virus; neuraminidase.
Conflict of interest statement
Heun-Soo Kang is an employee at Metabolab. However, the founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; and in the decision to publish the results.
Figures
Figure 1
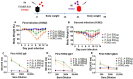
Fermented ginseng sample A showed higher antiviral protective activity against influenza viruses. Groups of mice (n = 5, wild-type BALB/c mice) were intranasally (IN) infected with a mixture of 1.5 LD50 A/Vietnam/1203/2004 (rgH5N1) and fermented ginseng sample A or B at different doses (250 μg and 500 μg). After 14 days of body weight monitoring, the serum sample was collected to detect the levels of IgG and IgG isotypes using a ELISA method. The mice that survived from the first rgH5N1 infection were challenged again with a lethal dose of rgH5N1 (3 LD50). (A) Body weight change after the first infection (rgH5N1) and (B) body weight change after the second infection (rgH5N1). (C) rgH5N1-specific IgG, (D) IgG1, and (E) IgG2a after the first infection. F.G.A: rgH5N1 virus + fermented ginseng sample A; F.G.B: rgH5N1 virus + fermented ginseng sample B; non-F.G: not fermented ginseng; rgH5N1 only: virus infection without ginseng samples.
Figure 2
Low dose of fermented ginseng extract sample A provided protection against rgH5N1 influenza virus during primary and secondary infection. Groups of mice (n = 5, wild-type C57BL/6) mice were intranasally (IN) infected with a mixture of 10 LD50 of A/Vietnam/1203/2004 (rgH5N1) virus and fermented ginseng sample A or B at different doses (20, 50, 100, 500 μg). After 14 days of body weight monitoring, the serum sample was collected to detect the levels of IgG and IgG isotypes using an ELISA method. The mice that survived from the first infection were challenged again with rgH5N1 (10 LD50). (A) Body weight change after the first infection (10 LD50, rgH5N1) and (B) body weight change after the second infection (15 LD50, rgH5N1). (C) rgH5N1-specific IgG, (D) IgG1, and (E) IgG2c after the first infection. F.B.A: rgH5N1 virus + fermented ginseng sample A; F.G.B: H5N1 virus + fermented ginseng sample B; rgH5N1 only: virus infection without ginseng samples.
Figure 3
Fermented ginseng sample A showed higher antiviral protective activity against 1.0 LD50 H3N2 influenza viruses. Groups of mice (n = 5, CD8 T cell-deficient, CD8KO) were intranasally (IN) infected with a mixture of 1 LD50 of A/Philippines/82 (H3N2) virus and fermented ginseng sample A or B at different doses (250 and 500 μg). After 14 days of body weight monitoring, the serum sample was collected to detect the levels of IgG and IgG isotypes after the first infection using an ELISA method. The mice that survived from H3N2 virus infection were challenged with homogenous virus (1.5 LD50, H3N2). (A) Body weight change after the first infection (H3N2) and (B) body weight change after the second infection (H3N2). (C) H3N2 specific IgG, (D) IgG1, and (E) IgG2c after the first infection. F.G.A: H3N2 virus + fermented ginseng sample A; F.G.B: H3N2 virus + fermented ginseng sample B; H3N2 only: virus infection without ginseng samples.
Figure 4
Fermented ginseng sample A had antiviral protective effects against H1N1 or rgH5N1 influenza virus even in severe immune-deficient mice. Groups of wild-type (n = 3, C57BL/6) and mutant mice (n = 3, B cell-deficient and MHCIIKO) were intranasally (IN) infected with a mixture of virus (2 LD50) and fermented ginseng sample A at different doses (250 and 500 μg). Body weight changes were monitored for 14 days after the infection. (A) A/Shanghai/2013 (rgH7N9, 2 LD50) virus inoculation in wild-type mice; (B) A/California/2009 (H1N1) virus inoculation in µMT (B cell deficient) mice; (C) A/Vietnam/1203/2004 (rgH5N1) virus inoculation in MHCIIKO mice. F.G.A: fermented ginseng sample A; rgH7N9 only: virus infection without ginseng samples; H1N1 only: virus infection without ginseng samples; rgH5N1 only: virus infection without ginseng samples.
Figure 5
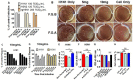
In vivo protection against A/WSN/1933 (H1N1) virus in wild-type BALB/c mice after inoculation with a mixture of fermented ginseng extracts and the virus. Groups of mice (n = 5) were infected with a mixture of A/WSN H1N1 virus and fermented ginseng sample A (250 μg). Lung viral titers were determined in the lung extracts at day 6 postinfection. (A) F.G.A 250 μg: mixed 250 μg of F.G.A with A/WSN (1× LD50) influenza virus; Ginseng only: no WSN virus, just mock infection with 1 mg F.G.A; F.G.A 1 mg postinfection: mice infected with WSN H1N1 first, treated with 1 mg F.G.A one hour postinfection, then treated with 1 mg every 1 h, for five times; H1N1 WSN only: virus infection only. (B) Lung viral titers from the mice at day 6 postinfection. (C) ELISA for IL-6 in lung. (D) Lung histopathology. Mock: no virus, no ginseng; F.G.A: F.G.A 1 mg postinfection; WSN H1N1 only: same as the description above. Magnification: 100×, Scale bars: 50 μm. Ginseng only: inoculation with ginseng, no viruses. *** indicates statistical significance, p < 0.0005 between F.G.A and comparing groups.
Figure 6
Fermented ginseng extract sample A had antiviral activity against influenza virus in vitro. (A) Microneutralization assay. Different strains of viruses (100 TCID50 H1N1, 120 TCID50 H3N2, and 200 TCID50 H5N1) were incubated with 10 mg/mL of F.G.A or F.G.B for 1 h at 37 °C and added to MDCK cells (1 × 105) for 18 h cultures. ELISA was done using primary antibody (Anti-NP) and secondary antibody (IgG-HRP) to determine percentage of growth based on control virus infection without ginseng samples. TCID50: Tissue culture infectious dose. (B) Plaque assay in MDCK cells. The H1N1 and F.G.A or F.G.B mixture incubated with MDCK cells monolayer for 30 min, then covered with 1.5% agarose to culture for 3–5 days to determine percentage of control plaques. (C) Effects of fermented ginseng samples treatment prior to infection on viral growth in plaques. The mixture of H1N1 California/A/2009 and 10 mg/mL of F.G.A or F.G.B was incubated for 0, 5, 10, and 20 min at 37 °C. (D) Effects of fermented ginseng samples treatment postinfection on viral growth in plaques. MDCK monolayers were first infected with H1N1 or H5N1 virus and incubated for 1 h at 37 °C. After washing away the virus, 10 mg/mL of F.G.A or F.G.B were added to virus-infected MDCK monolayers for 3 h or 6 h. (E,F) ELISA for F.G.A-treated viral plates. The 96-wells were coated with H1N1 or H5N1 inactivated virus overnight, then incubated with 10 mg of F.G.A or nonfermented ginseng for 1 h at 37 °C. The primary Anti-H1 HA and Anti-H5 HA and secondary goat anti-mouse IgG were used for ELISA test. (G) 8000 TCID50 of rgH5N1 virus was incubated with different concentration of F.G.A or F.G.B for 30 min, and neuraminidase activity was determined. (H) Cell viability test. 2 mL of EMEM medium (with 5 mg and 10 mg/mL of F.G.A) was incubated with MDCK monolayer for 6 h. Medium–ginseng mixture was removed, and cell viability was counted by trypan blue method. *, **, *** symbols indicate statistical significance, p < 0.05, p< 0.01 and p < 0.001 between the comparing groups. ‘ns’ indicates no significance.
Similar articles
- Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice.
Yoo DG, Kim MC, Park MK, Song JM, Quan FS, Park KM, Cho YK, Kang SM. Yoo DG, et al. J Med Food. 2012 Oct;15(10):855-62. doi: 10.1089/jmf.2012.0017. Epub 2012 Aug 2. J Med Food. 2012. PMID: 22856395 Free PMC article. - Greater Efficacy of Black Ginseng (CJ EnerG) over Red Ginseng against Lethal Influenza A Virus Infection.
Kim EH, Kim SW, Park SJ, Kim S, Yu KM, Kim SG, Lee SH, Seo YK, Cho NH, Kang K, Soung DY, Choi YK. Kim EH, et al. Nutrients. 2019 Aug 13;11(8):1879. doi: 10.3390/nu11081879. Nutrients. 2019. PMID: 31412594 Free PMC article. - Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase.
Ding Y, Dou J, Teng Z, Yu J, Wang T, Lu N, Wang H, Zhou C. Ding Y, et al. Arch Virol. 2014 Dec;159(12):3269-78. doi: 10.1007/s00705-014-2192-2. Epub 2014 Jul 31. Arch Virol. 2014. PMID: 25078390 - Compounds with anti-influenza activity: present and future of strategies for the optimal treatment and management of influenza. Part II: Future compounds against influenza virus.
Gasparini R, Amicizia D, Lai PL, Bragazzi NL, Panatto D. Gasparini R, et al. J Prev Med Hyg. 2014 Dec;55(4):109-29. J Prev Med Hyg. 2014. PMID: 26137785 Free PMC article. Review. - Emerging antiviral strategies to interfere with influenza virus entry.
Vanderlinden E, Naesens L. Vanderlinden E, et al. Med Res Rev. 2014 Mar;34(2):301-39. doi: 10.1002/med.21289. Epub 2013 Jun 25. Med Res Rev. 2014. PMID: 23801557 Free PMC article. Review.
Cited by
- In Vitro Investigations into the Potential Drug Interactions of Pseudoginsenoside DQ Mediated by Cytochrome P450 and Human Drug Transporters.
Li Z, Wang C, Liu J, Li P, Feng H. Li Z, et al. Molecules. 2024 May 24;29(11):2482. doi: 10.3390/molecules29112482. Molecules. 2024. PMID: 38893358 Free PMC article. - Antiviral activities of ginseng and its potential and putative benefits against monkeypox virus: A mini review.
Chandra Das R, Ratan ZA, Rahman MM, Runa NJ, Mondal S, Konstantinov K, Hosseinzadeh H, Cho JY. Chandra Das R, et al. J Ginseng Res. 2023 Apr 1;47(6):687-93. doi: 10.1016/j.jgr.2023.03.002. Online ahead of print. J Ginseng Res. 2023. PMID: 37362081 Free PMC article. Review. - Antiviral Potential of the Genus Panax: An updated review on their effects and underlying mechanism of action.
Zhang Y, Zhong X, Xi Z, Li Y, Xu H. Zhang Y, et al. J Ginseng Res. 2023 Mar;47(2):183-192. doi: 10.1016/j.jgr.2022.11.003. Epub 2022 Nov 17. J Ginseng Res. 2023. PMID: 36926608 Free PMC article. Review. - Antiviral Effect of Ginsenosides rk1 against Influenza a Virus Infection by Targeting the Hemagglutinin 1-Mediated Virus Attachment.
Yang X, Sun H, Zhang Z, Ou W, Xu F, Luo L, Liu Y, Chen W, Chen J. Yang X, et al. Int J Mol Sci. 2023 Mar 4;24(5):4967. doi: 10.3390/ijms24054967. Int J Mol Sci. 2023. PMID: 36902398 Free PMC article. - Novel and Alternative Therapeutic Strategies for Controlling Avian Viral Infectious Diseases: Focus on Infectious Bronchitis and Avian Influenza.
Abbas G, Yu J, Li G. Abbas G, et al. Front Vet Sci. 2022 Jul 22;9:933274. doi: 10.3389/fvets.2022.933274. eCollection 2022. Front Vet Sci. 2022. PMID: 35937298 Free PMC article. Review.
References
- CDC Disease Burden of Influenza. [(accessed on 1 August 2018)]; Available online: www.cdc.gov/flu/about/disease/burden.htm.
- Centers for Disease Control and Prevention Estimates of deaths associated with seasonal influenza—United States, 1976–2007. Morb. Mortal. Wkly. Rep. 2010;59:1057–1062. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials