Ring nucleases deactivate type III CRISPR ribonucleases by degrading cyclic oligoadenylate - PubMed (original) (raw)
Ring nucleases deactivate type III CRISPR ribonucleases by degrading cyclic oligoadenylate
Januka S Athukoralage et al. Nature. 2018 Oct.
Abstract
The CRISPR system provides adaptive immunity against mobile genetic elements in prokaryotes, using small CRISPR RNAs that direct effector complexes to degrade invading nucleic acids1-3. Type III effector complexes were recently demonstrated to synthesize a novel second messenger, cyclic oligoadenylate, on binding target RNA4,5. Cyclic oligoadenylate, in turn, binds to and activates ribonucleases and other factors-via a CRISPR-associated Rossman-fold domain-and thereby induces in the cell an antiviral state that is important for immunity. The mechanism of the 'off-switch' that resets the system is not understood. Here we identify the nuclease that degrades these cyclic oligoadenylate ring molecules. This 'ring nuclease' is itself a protein of the CRISPR-associated Rossman-fold family, and has a metal-independent mechanism that cleaves cyclic tetraadenylate rings to generate linear diadenylate species and switches off the antiviral state. The identification of ring nucleases adds an important insight to the CRISPR system.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures
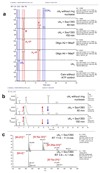
Extended Data Figure 1. Genome organisation of the CRISPR-Cas locus of S. solfataricus
The type I-A, III-B and III-D effector complex operons are depicted, along with genes encoding adaptation proteins and the position of the six CRISPR loci (A-F). Genes outlined in blue encode proteins with CARF domains. The structures of three CARF family proteins are shown with CARF domains coloured green, along with the structure of cA4.
Extended Data Figure 2. Structure-guided sequence alignment of Sso1393, Sso2081 and orthologues.
Multiple sequence alignment showing Sso1393, together with three homologues, aligned with Sso2081 and three homologues. Secondary structure is shown above the alignment, based on the structure of Sso1393 (PDB 3QYF). Ser-11 and Lys-168 are indicated by asterisks. Conserved residues are shaded. Sequences aligned are from S. solfataricus (Sso1393, Sso2081); S. islandicus REY15A (SiRE_0811, SiRE_0455); S. islandicus M.16.4 (M164_0884); S. acidocaldarius (Saci_1063) and S. tokodaii (Stk_17430).
Extended Data Figure 3. Single-turnover kinetic analysis of cA4 degradation by a, Sso2081 and b, Sso1393.
Representative phosphorimages of denaturing polyacrylamide gel electrophoresis (PAGE) analysing the reaction of radioactively labelled cA4 with a, 2 μM Sso2081 or b, 2 μM Sso1393 at 60 °C over time. Triplicate experiments were carried out, quantified by phosphorimaging and the fraction of cA4 cleaved plotted in Figure 3b.
Extended Data Figure 4. Sso2081 and Sso1393 cA4 degradation mechanism investigated by TLC.
Csm generated cOA (lane 1) was incubated with 2 μM Sso2081 dimer at 60 °C to determine the intermediate (Y) and final (X) reaction product over time (lanes 2-10). Lanes 11-13 show reaction product seen in lanes 2, 4 and 6, 5’-end phosphorylated using T4 polynucleotide kinase (PNK) for identification of reaction intermediates and products by comparison to 5’-end phosphorylated MazF nuclease generated HO-A2>P and HO-A4>P standards. Lanes 14 & 15 show the reaction products of 2 μM Sso1393 dimer incubated with cOA at 70 °C for 20 and 120 min, respectively. Reaction product from lanes 14 & 15 are 5’-end phosphorylated by PNK for comparison to P-A2>P and P-A4>P standards. Comparison of PNK treated reaction product to standards showed the presence of a low amount of intermediate (P-Y) during the Sso2081 cA4 cleavage reaction, which migrated similarly to the P-A4>P standard and did not change in abundance over time, whereas the abundance of the final product (P-X) increased over time. In contrast, comparison of Sso1393 PNK treated 20 min and 120 min reaction products showed a decrease of the intermediate (P-Y) over time and increase of product (P-X).
Extended Data Figure 5. LC-MS analysis of Sso2081 reactions.
a, Ion chromatograms extracted for m/z 657.1 (cA2-1, A2>P-1, cA4-2, A4>P-2). Individual lanes are labelled. cOAs (mainly cA4), derived from reaction of Csm with ATP, was incubated with Sso2081 for 60 and 150 min. Linear oligoadenylates with 2’,3’-cyclic phosphate were derived from hydrolysis of A4 RNA oligonucleotide with MazF. b, UV traces at 254 nm. Peaks that change in intensity over the course of the enzymatic reaction are indicated by arrows. The three peaks that decreased or increased over the course of the reaction all match the changes in abundance of the m/z 657.1 species. No changes are observed after 60 min reaction time. The broad peak at 4.9 min is an unknown contaminant likely resulting from the phenol-chloroform extraction. Shifts in retention time are possibly due to matrix effects. c, Mass spectra for cA4 and product X. Calculated for C20H23N10O12P2-1 (cA2 / A2>P) m/z 657.0978, found 657.0966 (_δ_m -1.8 ppm); calculated for C40H46N20O24P4-2 (cA4 / A4>P) m/z 657.0978, found 657.0967 (_δ_m -1.6 ppm). The data presented are representative of duplicate experiments.
Extended Data Figure 6. LC-MS analysis of Sso1393 reactions.
a, Ion chromatograms extracted for m/z 657.1 (cA2-1, A2>P-1, cA4-2, A4>P-2). Individual lanes are labeled. cOAs (mainly cA4), derived from reaction of Csm with ATP, was incubated with Sso1393 for 60 and 150 min or without Ring nuclease for 150 min. A control in which the ATP from the Csm cyclase reaction had been omitted was also analysed as control. Linear oligoadenylates with 2’,3’-cyclic phosphate were derived from hydrolysis of suitable DNA oligonucleotide subtrates with the toxin MazF; whereas cA2 was a commercially available standard. The traces show clearly the difference in retention time between the linear and cyclic isomers. b, UV traces at 254 nm. Peaks that change in intensity over the course of the enzymatic reaction are indicated by arrows. The three peaks that decreased or increased over the course of the reaction all match the changes in abundance of the m/z 657.1 species. The broad peak at 4.1 – 6 min is an unknown contaminant likely resulting from the phenol-chloroform extraction. c, Mass spectra for species X and Y. Calculated for C20H23N10O12P2-1 (cA2 / A2>P) m/z 657.0978, found 657.0968 (_δ_m -1.4 ppm); calculated for C40H46N20O24P4-2 (cA4 / A4>P) m/z 657.0978, found 657.0967 (_δ_m -1.6 ppm). The data presented are representative of duplicate experiments.
Extended Data Figure 7. Specificity of Sso2081 for cyclic nucleotide substrates.
Various cyclic di- and oligonucleotides were incubated in the absence (ctrl) or presence of Sso2081 as described in Materials and Methods. Dinucleotides and cA6 were obtained from BIOLOG Life Science Institute (Bremen), cA4 was obtained from an enzymatic reaction with S. solfataricus Csm. Protein-free extracts were analyzed by LC-HRMS essentially as before but using a shorter column and gradient. EICs for substrate and expected products are shown in each panel. No reaction was observed for any of the cyclic dinucleotides. cA4, was completely converted to linear A2>P by Sso2081 whilst only a small percentage of cA6 was converted, demonstrating a clear preference of Sso2081 for its physiological substrate cA4. The data presented are representative of duplicate experiments.
Extended Data Figure 8. Structure and kinetic analysis of Sso1393 and Sso2081.
a, Structure of Sso1393 (PDB 3QYF), with cA4 docked at the active site. This is an orthogonal view to that shown in Fig. 3. The CARF domain is coloured green. The side chains of Ser-11 and Lys-168 are shown. b, Model of the Sso2081 structure. Only the CARF domain (aa1-125) is modelled. Conserved residues Ser-11, Arg-105 and Lys-106 are labelled. Model generated using Phyre. c, Representative phosphorimages of denaturing PAGE assessing the cA4 degradation activity of Sso2081 compared to its catalytically inactive R105A/K106A mutant over time (left hand panel), and Sso1393 activity compared to its K168A mutant (right hand panel). All reactions were carried out at 70 °C with 2 μM protein dimer. The data presented are representative of triplicate experiments. d, Single-turnover kinetics of Sso2081 and Sso1393 plotted alongside their S11A active site mutants and fitted to an exponential equation. Rate constants are displayed with the legend (Sso2081; 0.23 ± 0.01 min-1, Sso2081 S11A; 0.066 ± 0.002 min-1, Sso1393; 0.024 ± 0.0003 min-1, Sso1393 S11A; 0.00076 ± 0.001 min-1). Experiments were carried out in triplicate with means and standard deviation shown. The data presented are technical replicates and are representative of duplicate experiments.
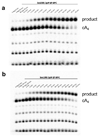
Extended Data Figure 9. Ring nucleases abrogate activation of the Csx1 nuclease by converting cA4 to A2>P.
a, In the absence of cA4, Csx1 had no ribonuclease activity (lane c), but addition of cA4 resulted in degradation of the substrate RNA molecule. When cA4 was pre-incubated for 1 h at 70 °C with Sso2081 in buffer E before addition to the assay, Csx1 ribonuclease activity was abolished. Mock treatment using buffer instead of Sso2081 (denoted ‘*’) had no effect. b, As for a, but pre-incubated with Sso1393 for 2 h at 70 °C. c, Using radioactive cA4, the deactivation of Csx1 by Sso2081 and Sso1393 was observed to correlate with the conversion of cA4 into A2>P. Results shown are representative of at least triplicate experiments.
Figure 1. Purification and identification of the enzyme that degrades cA4
S. solfataricus cell lysate was fractionated by a, phenyl-sepharose, b, size exclusion and c, heparin chromatography. At each stage, fractions were assayed for cA4 conversion activity using radioactive cA4, and active fractions generating product X (indicated by shaded boxes) were pooled for the next stage. d, Following the heparin column, each fraction was assayed and analysed by SDS-PAGE. The band corresponding to the peak of activity (arrowed) was excised from the gel and identified by mass spectrometry as Sso2081, a CARF domain protein. e, Purified recombinant Sso2081 and 1393 degrade cA4, but Csx1 does not. f, Only Csx1 degrades linear RNA in the presence of cA4. Panels a-f are representative of at least duplicate experiments.
Figure 2. Kinetics and products of Ring nuclease activity
a, Single turnover kinetic analysis of cA4 cleavage by Sso2081 and Sso1393. Sso2081 is a 10-fold faster enzyme. Data points are the means of triplicate measurements, with standard deviation shown, and are technical replicates representative of duplicate experiments. b, TLC analysis of substrates and products. Lane 1 shows cA4 synthesised by the Csm complex. Lane 2 shows the product (X) of conversion by Sso2081 after 20 min. Lane 3 shows product (X) following phosphorylation by PNK. Lanes 4-7 show the products (X and Y) of cA4 conversion by Sso1393 after 20 and 120 min, respectively, before and after PNK treatment. Lanes 8 and 9 are markers for P-A2>P and P-A4>P generated by the MazF nuclease. This gel is representative of duplicate experiments. c, d, LC-HRMS analysis of cA4 cleavage by Sso2081 and Sso1393, respectively, showing ion chromatograms extracted for m/z 657.10 ± 0.5 corresponding to cA4-2, A4>P-2 and A2>P-1. The control is cA4 incubated for 150 min without enzyme. Traces for the conversion of cA4 by Sso2081 after 60 min, and by Sso1393 after 60 and 150 min are shown. Products X and Y are indicated. The bottom traces show the linear oligoadenylates A2>P and A4>P, generated by MazF, as standards. The data are representative of at least duplicate experiments.
Figure 3. Structure and mechanism of Ring nucleases
a, Structure of the CARF domain of Sso1393 with cA4 docked. The active site residues K168 and S11 are shown. b, Kinetic analysis of Sso1393 (wild-type, S11A and K168A variants) and Sso2081 (wild-type, S11A and R105A/K106A variants). **Catalytic rate constants under single turnover conditions are plotted. The data points are derived from exponential fits to the triplicate rate measurements presented in**Extended Data Fig. 8, with the standard errors derived from curve fitting shown. c, Cartoon showing the reaction scheme for conversion of cyclic to linear A4>P and A2>P. S11 may participate in the correct positioning of the 2’-OH group of the ribose to facilitate nucleophilic attack, whilst the basic residue K168 (and R105/K106 in Sso2081) may stabilise the penta-covalent phosphorus formed in the transition state.
Figure 4. Reconstitution of the cOA signalling pathway
a, The Csm effector generates cA4 in proportion to the amount of viral target RNA present , activating the HEPN nuclease Csx1. The presence of Sso2081 (2.5 µM) for 1 h prior to the addition of Csx1 (0.5 µM) for 20 min at 70 °C reversed Csx1 activation partially when 10 nM target RNA was present, and fully when lower amounts of RNA were used. Control c shows the experiment in the presence of Sso2081 and absence of target RNA. Levels of cA4 and A2>P were monitored by TLC. The data are representative of three separate experiments. b, Schematic of the cOA signalling system. Type III effectors loaded with crRNA bind to viral target RNA, activating the HD nuclease, which targets viral DNA, and the Cyclase domain. cOA is synthesised, activating the Csx1 ribonuclease. Target RNA cleavage and subsequent dissociation deactivates the Cyclase domain, stopping synthesis of cOA. The Ring nucleases complete the deactivation of the antiviral state by degrading extant cOA.
Comment in
- If You'd Like to Stop a Type III CRISPR Ribonuclease, Then You Should Put a Ring (Nuclease) on It.
Mo CY, Marraffini LA. Mo CY, et al. Mol Cell. 2018 Nov 15;72(4):608-609. doi: 10.1016/j.molcel.2018.10.048. Mol Cell. 2018. PMID: 30444997
Similar articles
- Structural basis of cyclic oligoadenylate degradation by ancillary Type III CRISPR-Cas ring nucleases.
Molina R, Jensen ALG, Marchena-Hurtado J, López-Méndez B, Stella S, Montoya G. Molina R, et al. Nucleic Acids Res. 2021 Dec 2;49(21):12577-12590. doi: 10.1093/nar/gkab1130. Nucleic Acids Res. 2021. PMID: 34850143 Free PMC article. - Investigation of the cyclic oligoadenylate signaling pathway of type III CRISPR systems.
Rouillon C, Athukoralage JS, Graham S, Grüschow S, White MF. Rouillon C, et al. Methods Enzymol. 2019;616:191-218. doi: 10.1016/bs.mie.2018.10.020. Epub 2019 Jan 12. Methods Enzymol. 2019. PMID: 30691643 - Regulation of cyclic oligoadenylate synthesis by the Staphylococcus epidermidis Cas10-Csm complex.
Nasef M, Muffly MC, Beckman AB, Rowe SJ, Walker FC, Hatoum-Aslan A, Dunkle JA. Nasef M, et al. RNA. 2019 Aug;25(8):948-962. doi: 10.1261/rna.070417.119. Epub 2019 May 10. RNA. 2019. PMID: 31076459 Free PMC article. - Hot and crispy: CRISPR-Cas systems in the hyperthermophile Sulfolobus solfataricus.
Zhang J, White MF. Zhang J, et al. Biochem Soc Trans. 2013 Dec;41(6):1422-6. doi: 10.1042/BST20130031. Biochem Soc Trans. 2013. PMID: 24256231 Review. - Cutting it close: CRISPR-associated endoribonuclease structure and function.
Hochstrasser ML, Doudna JA. Hochstrasser ML, et al. Trends Biochem Sci. 2015 Jan;40(1):58-66. doi: 10.1016/j.tibs.2014.10.007. Epub 2014 Nov 18. Trends Biochem Sci. 2015. PMID: 25468820 Review.
Cited by
- Virus-borne mini-CRISPR arrays are involved in interviral conflicts.
Medvedeva S, Liu Y, Koonin EV, Severinov K, Prangishvili D, Krupovic M. Medvedeva S, et al. Nat Commun. 2019 Nov 15;10(1):5204. doi: 10.1038/s41467-019-13205-2. Nat Commun. 2019. PMID: 31729390 Free PMC article. - Type III CRISPR-Cas Systems: Deciphering the Most Complex Prokaryotic Immune System.
Kolesnik MV, Fedorova I, Karneyeva KA, Artamonova DN, Severinov KV. Kolesnik MV, et al. Biochemistry (Mosc). 2021 Oct;86(10):1301-1314. doi: 10.1134/S0006297921100114. Biochemistry (Mosc). 2021. PMID: 34903162 Free PMC article. Review. - The CRISPR ancillary effector Can2 is a dual-specificity nuclease potentiating type III CRISPR defence.
Zhu W, McQuarrie S, Grüschow S, McMahon SA, Graham S, Gloster TM, White MF. Zhu W, et al. Nucleic Acids Res. 2021 Mar 18;49(5):2777-2789. doi: 10.1093/nar/gkab073. Nucleic Acids Res. 2021. PMID: 33590098 Free PMC article. - Type III-A CRISPR-associated protein Csm6 degrades cyclic hexa-adenylate activator using both CARF and HEPN domains.
Smalakyte D, Kazlauskiene M, F Havelund J, Rukšėnaitė A, Rimaite A, Tamulaitiene G, Færgeman NJ, Tamulaitis G, Siksnys V. Smalakyte D, et al. Nucleic Acids Res. 2020 Sep 18;48(16):9204-9217. doi: 10.1093/nar/gkaa634. Nucleic Acids Res. 2020. PMID: 32766806 Free PMC article. - The RNA repair proteins RtcAB regulate transcription activator RtcR via its CRISPR-associated Rossmann fold domain.
Kotta-Loizou I, Giuliano MG, Jovanovic M, Schaefer J, Ye F, Zhang N, Irakleidi DA, Liu X, Zhang X, Buck M, Engl C. Kotta-Loizou I, et al. iScience. 2022 Oct 20;25(11):105425. doi: 10.1016/j.isci.2022.105425. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388977 Free PMC article.
References
- Mojica FJ, Rodriguez-Valera F. The discovery of CRISPR in archaea and bacteria. FEBS J. 2016;283:3162–3169. - PubMed
- Niewoehner O, et al. Type III CRISPR-Cas systems produce cyclic oligoadenylate second messengers. Nature. 2017;548:543–548. - PubMed
- Kazlauskiene M, Kostiuk G, Venclovas C, Tamulaitis G, Siksnys V. A cyclic oligonucleotide signaling pathway in type III CRISPR-Cas systems. Science. 2017;357:605–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/G011400/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M000400/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/M021017/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources