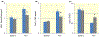Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes - PubMed (original) (raw)
Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes
Tsuneo Takenaka et al. Acta Physiol (Oxf). 2019 Feb.
Abstract
Aims: Klotho interacts with various membrane proteins, such as receptors for transforming growth factor (TGF)-β and insulin-like growth factor (IGF), to alter their function. Renal expression of klotho is diminished in diabetes. The present study examined whether exogenous klotho protein supplementation ameliorates kidney injury and renin-angiotensin system (RAS) in db/db mice.
Methods: We investigated the effects of klotho supplementation on diabetic kidney injury and RAS. Recombinant human klotho protein (10 μg/kg/d) was administered to db/db mice daily.
Results: Klotho protein supplementation reduced kidney weight, systolic blood pressure (SBP), albuminuria, glomerular filtration rate, and 8-epi-prostaglandin F2α excretion without affecting body weight. Although klotho supplementation did not alter glycated albumin, it reduced renal angiotensin II levels associated with reduced renal expression of angiotensinogen. Klotho supplementation improved renal expression of superoxide dismutase (SOD), and endogenous renal expression of klotho. Klotho supplementation reduced the levels of hypoxia-inducible factor, phosphorylated Akt, and phosphorylated mTOR and decreased the renal expression of TGF-β, tumour necrosis factor (TNF), and fibronectin.
Conclusions: These data indicate that klotho supplementation reduces blood pressure and albuminuria along with ameliorating renal RAS activation in db/db mice. Furthermore, these results suggest that klotho inhibits IGF signalling, induces SOD expression to reduce oxidative stress, and suppresses Akt-mTOR signalling to inhibit abnormal kidney growth. Collectively, the results suggest that klotho inhibits TGF-β and TNF signalling, resulting in a decline in renal fibrosis.
Keywords: epithelial-mesenchymal transition; insulin-like growth factor; mTOR; superoxide dismutase; transforming growth factor; tumour necrosis factor.
© 2018 Scandinavian Physiological Society. Published by John Wiley & Sons Ltd.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no conflicts of interest regarding this manuscript.
Figures
FIGURE 1
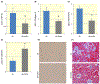
Impact of exogenous klotho protein supplementation on renal expressions of TGF-β (A), collagen I (B), fibronectin (C), and E-cadherin (D), Smad3 distribution (E), and interstitial fibrosis (F) in db/db mice (db). The * indicates statistically significant differences between the two groups (n = 10 for each)
FIGURE 2
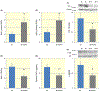
Influences of exogenous klotho protein supplementation on phosphorylation of Akt (A, 56 kDa), mTOR (B, 289 kDa), and p70-S6k (C, 70 kDa), and phosphorylated mTOR staining (D) in db/db mice (db). The * indicates statistically significant differences between the two groups (n = 10 for each). db + k depicts db/db mice with klotho supplementation
FIGURE 3
Effects of exogenous klotho protein supplementation on aortic (A) and renal (B) expressions of superoxide dismutase (SOD), renal abundance of hypoxia-inducible factor-1α (C, 110 kDa, HIF-1α), renal expression of tumour necrosis factor-α (D, TNF-α), plasma concentration of TNF-α (E), and phosphorylation of Iκβ (F, 36 kDa) in db/db mice (db). β-actin was observed at 42 kDa. The * indicates statistically significant differences between the two groups (n = 10 for each). db + k depicts db/db mice with klotho supplementation
FIGURE 4
Summary of in vitro studies in HK-2 cells. Hydrogen peroxide induced angiotensinogen expression (A) and klotho suppressed this response (For time: F = 36, df = 1, P < 0.005; for klotho treatment: F = 14, df = 1, P < 0.001; for interaction: F = 5, df = 1, P < 0.05; for error: df = 20). An interaction between time and klotho treatment may relate to transcytosis of klotho protein by proximal tubular cells. Similarly, hydrogen peroxide induced the expression of tumour necrosis factor-α (B, TNF-alpha), and klotho inhibited this (For time: F = 85, df = 1, P < 0.001; for klotho treatment: F = 8, df = 1, P < 0.05; for interaction: F = 13, df = 1, P < 0.01; for error: df = 20). Insulin-like growth factor repressed expression of superoxide dismutase (C, SOD), and klotho opposed this response (For time: F = 96, df = 1, P < 0.001; for klotho treatment: F = 6, df = 1, P < 0.05; for interaction: F = 9, df = 1, P < 0.01; for error: df = 20). Blue and grey bars depict control and klotho-treated groups respectively. The * indicates statistically significant differences between the two groups
Similar articles
- Klotho supplementation ameliorates blood pressure and renal function in DBA/2-pcy mice, a model of polycystic kidney disease.
Takenaka T, Kobori H, Inoue T, Miyazaki T, Suzuki H, Nishiyama A, Ishii N, Hayashi M. Takenaka T, et al. Am J Physiol Renal Physiol. 2020 Mar 1;318(3):F557-F564. doi: 10.1152/ajprenal.00299.2019. Epub 2020 Jan 13. Am J Physiol Renal Physiol. 2020. PMID: 31928223 - Klotho supplementation attenuates blood pressure and albuminuria in murine model of IgA nephropathy.
Takenaka T, Hasan A, Marumo T, Kobori H, Inoue T, Miyazaki T, Suzuki H, Nishiyama A, Ishii N, Hayashi M. Takenaka T, et al. J Hypertens. 2021 Aug 1;39(8):1567-1576. doi: 10.1097/HJH.0000000000002845. J Hypertens. 2021. PMID: 33758157 - Klotho Ameliorates Medullary Fibrosis and Pressure Natriuresis in Hypertensive Rat Kidneys.
Takenaka T, Inoue T, Miyazaki T, Kobori H, Nishiyama A, Ishii N, Hayashi M, Suzuki H. Takenaka T, et al. Hypertension. 2018 Nov;72(5):1151-1159. doi: 10.1161/HYPERTENSIONAHA.118.11176. Hypertension. 2018. PMID: 30354813 Free PMC article. - Recent insights into diabetic renal injury from the db/db mouse model of type 2 diabetic nephropathy.
Tesch GH, Lim AK. Tesch GH, et al. Am J Physiol Renal Physiol. 2011 Feb;300(2):F301-10. doi: 10.1152/ajprenal.00607.2010. Epub 2010 Dec 8. Am J Physiol Renal Physiol. 2011. PMID: 21147843 Review. - Limitations and future treatment options in type 2 diabetes with renal impairment.
Ritz E. Ritz E. Diabetes Care. 2011 May;34 Suppl 2(Suppl 2):S330-4. doi: 10.2337/dc11-s242. Diabetes Care. 2011. PMID: 21525478 Free PMC article. Review. No abstract available.
Cited by
- A systematic review and meta-analysis demonstrating Klotho as an emerging exerkine.
Corrêa HL, Raab ATO, Araújo TM, Deus LA, Reis AL, Honorato FS, Rodrigues-Silva PL, Neves RVP, Brunetta HS, Mori MADS, Franco OL, Rosa TDS. Corrêa HL, et al. Sci Rep. 2022 Oct 20;12(1):17587. doi: 10.1038/s41598-022-22123-1. Sci Rep. 2022. PMID: 36266389 Free PMC article. - Association between serum Klotho concentration and all-cause and cardiovascular mortality among American individuals with hypertension.
Yan Y, Chen J. Yan Y, et al. Front Cardiovasc Med. 2022 Nov 15;9:1013747. doi: 10.3389/fcvm.2022.1013747. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36457804 Free PMC article. - Pathophysiology and genetics of salt-sensitive hypertension.
Maaliki D, Itani MM, Itani HA. Maaliki D, et al. Front Physiol. 2022 Sep 13;13:1001434. doi: 10.3389/fphys.2022.1001434. eCollection 2022. Front Physiol. 2022. PMID: 36176775 Free PMC article. Review. - Klotho inhibits IGF1R/PI3K/AKT signalling pathway and protects the heart from oxidative stress during ischemia/reperfusion injury.
Olejnik A, Radajewska A, Krzywonos-Zawadzka A, Bil-Lula I. Olejnik A, et al. Sci Rep. 2023 Nov 20;13(1):20312. doi: 10.1038/s41598-023-47686-5. Sci Rep. 2023. PMID: 37985893 Free PMC article. - Klotho: a potential therapeutic target in aging and neurodegeneration beyond chronic kidney disease-a comprehensive review from the ERA CKD-MBD working group.
Kanbay M, Copur S, Ozbek L, Mutlu A, Cejka D, Ciceri P, Cozzolino M, Haarhaus ML. Kanbay M, et al. Clin Kidney J. 2023 Nov 3;17(1):sfad276. doi: 10.1093/ckj/sfad276. eCollection 2024 Jan. Clin Kidney J. 2023. PMID: 38213484 Free PMC article.
References
- Alicic RZ, Tuttle KR. Novel therapies for diabetic kidney disease. Adv Chronic Kidney Dis. 2014;21(2):121–133. - PubMed
- Pergola PE, Raskin P, Toto RD, et al. Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med. 2011;365(4):327–336. - PubMed
- Inomata S Renal hypertrophy as a prognostic index for the progression of diabetic renal disease in non-insulin-dependent diabetes mellitus. J Diab Comp. 1993;7:28–33. - PubMed
- Kleinman KS, Fine LG. Prognostic implications of renal hypertrophy in diabetes mellitus. Diabetes Metab Rev. 1988;4:179–189. - PubMed
- Segev Y, Eshet R, Yakir O, Haim N, Phillip M, Landau D. Systemic and renal growth hormone-IGF1 axis involvement in a mouse model of type 2 diabetes. Diabetologia. 2007;50(6):1327–1334. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous