Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors - PubMed (original) (raw)
Neurochemical pharmacology of psychoactive substituted N-benzylphenethylamines: High potency agonists at 5-HT2A receptors
Amy J Eshleman et al. Biochem Pharmacol. 2018 Dec.
Abstract
The use of new psychoactive substituted 2,5-dimethoxy-N-benzylphenethylamines is associated with abuse and toxicity in the United States and elsewhere and their pharmacology is not well known. This study compares the mechanisms of action of 2-(2,5-dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe), 2-(4-ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25E-NBOMe), 2-(2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25H-NBOMe), 2-(((4-iodo-2,5-dimethoxyphenethyl)amino)methyl)phenol (25I-NBOH); and 2-(2,5-dimethoxy-4-nitrophenyl)-N-(2-methoxybenzyl)ethanamine) (25N-NBOMe) with hallucinogens and stimulants. Mammalian cells heterologously expressing 5-HT1A, 5-HT2A, 5-HT2B or 5-HT2C receptors, or dopamine, serotonin or norepinephrine transporters (DAT, SERT and NET, respectively) were used to assess drug affinities at radioligand binding sites. Potencies and efficacies were determined using [35S]GTPγS binding assays (5-HT1A), inositol-phosphate accumulation assays (5-HT2A, 5-HT2B and 5-HT2C), and uptake and release assays (transporters). The substituted phenethylamines were very low potency and low efficacy agonists at the 5-HT1A receptor. 25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH and 25N-NBOMe had very high affinity for, and full efficacy at, 5-HT2A and 5-HT2C receptors. In the 5-HT2A receptor functional assay, 25D-NBOMe, 25E-NBOMe, 25I-NBOH and 25N-NBOMe had subnanomolar to low nanomolar potencies similar to (+)lysergic acid diethylamide (LSD) while 25H-NBOMe had lower potency, similar to serotonin. At the 5-HT2C receptor, four had very high potencies, similar to LSD and serotonin, while 25H-NBOMe had lower potency. At the 5-HT2B receptor, the compounds had lower affinity, potency and efficacy compared to 5-HT2A or 5-HT2C. The phenethylamines had low to mid micromolar affinities and potencies at the transporters. These results demonstrate that these -NBOMe and -NBOH substituted phenethylamines have a biochemical pharmacology consistent with hallucinogenic activity, with little psychostimulant activity.
Keywords: 2-(((4-Iodo-2,5-dimethoxyphenethyl)amino)methyl)phenol (25I-NBOH) (PubChem CID: 10001761); 2-(2,5-Dimethoxy-4-methylphenyl)-N-(2-methoxybenzyl)ethanamine (25D-NBOMe) (PubChem CID: 118536027); 2-(2,5-Dimethoxy-4-nitrophenyl)-N-(2-methoxybenzyl)ethanamine) (25N-NBOMe) (PubChem CID: 118536028); 2-(2,5-Dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25H-NBOMe) (PubChem CID: 121230760); 2-(4-Ethyl-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25E-NBOMe) (PubChem CID: 121230757); Drug abuse; Lysergic acid diethylamide (LSD); NBOMe; Serotonin receptor; Substituted phenethylamine.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest
All authors declare that they have no conflicts of interest.
Figures
Figure 1.
Chemical structures of 25D-NBOMe, 25E-NBOMe, 25H-NBOMe, 25I-NBOH, 25N-NBOMe, 5-HT, LSD and DOM.
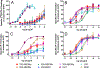
Figure 2.
Agonist activity of -NBOMe phenethylamines at recombinant 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors. All data were normalized to the maximal effect of 5-HT, which was measured on each experimental day. A. 5-HT1A [35S]GTPγS binding. N=3–7 independent experiments conducted with duplicate determinations. B. 5-HT2A agonist IP-1 assay. N=3–4 independent experiments conducted with duplicate determinations. C. 5-HT2B IP-1 assay. N=4–6 independent experiments conducted with duplicate determinations. D. 5-HT2C IP-1 assay. N=3–4 independent experiments conducted with duplicate determinations. Data shown are mean ± sem.
Figure 3.
Correlation of affinities and agonist potencies of substituted phenethylamines at 5-HT1A, 5-HT2A, 5-HT2B and 5-HT2C receptors. The linear regression for the data in each graph is shown. A. 5-HT1A affinities as measured with [3H]8-OH-DPAT binding vs 5-HT1A potencies as measured using [35S]GTPγS binding. Spearman r=0.19, p>0.05. B. 5-HT2A affinities as measured with [125I]DOI binding vs 5-HT2A potencies as measured using the IP-1 assay. Spearman r=0.72, p=0.01. C. 5-HT2B affinities as measured with [3H]5-HT binding vs 5-HT2B potencies as measured using the IP-1 assay. Spearman r=0.76, p<0.05. D. 5-HT2C affinities as measured with [125I]DOI binding vs 5-HT2C potencies as measured with IP-1 assay. Spearman r=0.67, p<0.05. Values for 2C-C, 2C-D, 2C-E, 2C-I, 2C-T-2 and DOC are from [23]
Similar articles
- Receptor interaction profiles of novel N-2-methoxybenzyl (NBOMe) derivatives of 2,5-dimethoxy-substituted phenethylamines (2C drugs).
Rickli A, Luethi D, Reinisch J, Buchy D, Hoener MC, Liechti ME. Rickli A, et al. Neuropharmacology. 2015 Dec;99:546-53. doi: 10.1016/j.neuropharm.2015.08.034. Epub 2015 Aug 25. Neuropharmacology. 2015. PMID: 26318099 - Comparative neuropharmacology of N-(2-methoxybenzyl)-2,5-dimethoxyphenethylamine (NBOMe) hallucinogens and their 2C counterparts in male rats.
Elmore JS, Decker AM, Sulima A, Rice KC, Partilla JS, Blough BE, Baumann MH. Elmore JS, et al. Neuropharmacology. 2018 Nov;142:240-250. doi: 10.1016/j.neuropharm.2018.02.033. Epub 2018 Mar 1. Neuropharmacology. 2018. PMID: 29501528 Free PMC article. - Pharmacology and Toxicology of N-Benzylphenethylamine ("NBOMe") Hallucinogens.
Halberstadt AL. Halberstadt AL. Curr Top Behav Neurosci. 2017;32:283-311. doi: 10.1007/7854_2016_64. Curr Top Behav Neurosci. 2017. PMID: 28097528 Review. - Behavioral and neurochemical pharmacology of six psychoactive substituted phenethylamines: mouse locomotion, rat drug discrimination and in vitro receptor and transporter binding and function.
Eshleman AJ, Forster MJ, Wolfrum KM, Johnson RA, Janowsky A, Gatch MB. Eshleman AJ, et al. Psychopharmacology (Berl). 2014 Mar;231(5):875-88. doi: 10.1007/s00213-013-3303-6. Epub 2013 Oct 19. Psychopharmacology (Berl). 2014. PMID: 24142203 Free PMC article. - Severe 25E-NBOH intoxication associated with MDPHP intake: a case report, metabolism study, and literature review.
Pelletier R, Gicquel T, Carvelli J, Balaz P, Pelissier-Alicot AL, Morel I, Bottinelli C, Solas C, Le Daré B, Fabresse N. Pelletier R, et al. Int J Legal Med. 2024 May;138(3):815-822. doi: 10.1007/s00414-023-03151-6. Epub 2023 Dec 20. Int J Legal Med. 2024. PMID: 38117418 Review.
Cited by
- Pharmacologic Activity of Substituted Tryptamines at 5-Hydroxytryptamine (5-HT)2A Receptor (5-HT2AR), 5-HT2CR, 5-HT1AR, and Serotonin Transporter.
Kozell LB, Eshleman AJ, Swanson TL, Bloom SH, Wolfrum KM, Schmachtenberg JL, Olson RJ, Janowsky A, Abbas AI. Kozell LB, et al. J Pharmacol Exp Ther. 2023 Apr;385(1):62-75. doi: 10.1124/jpet.122.001454. Epub 2023 Jan 20. J Pharmacol Exp Ther. 2023. PMID: 36669875 Free PMC article. - Molecular and Medical Aspects of Psychedelics.
Wojtas A, Gołembiowska K. Wojtas A, et al. Int J Mol Sci. 2023 Dec 23;25(1):241. doi: 10.3390/ijms25010241. Int J Mol Sci. 2023. PMID: 38203411 Free PMC article. Review. - Serotonin 2A Receptor (5-HT2AR) Activation by 25H-NBOMe Positional Isomers: In Vitro Functional Evaluation and Molecular Docking.
Pottie E, Kupriyanova OV, Brandt AL, Laprairie RB, Shevyrin VA, Stove CP. Pottie E, et al. ACS Pharmacol Transl Sci. 2021 Feb 25;4(2):479-487. doi: 10.1021/acsptsci.0c00189. eCollection 2021 Apr 9. ACS Pharmacol Transl Sci. 2021. PMID: 33860178 Free PMC article. - Acute DOB and PMA Administration Impairs Motor and Sensorimotor Responses in Mice and Causes Hallucinogenic Effects in Adult Zebrafish.
Tirri M, Ponzoni L, Bilel S, Arfè R, Braida D, Sala M, Marti M. Tirri M, et al. Brain Sci. 2020 Aug 24;10(9):586. doi: 10.3390/brainsci10090586. Brain Sci. 2020. PMID: 32847111 Free PMC article. - 5-HT2 receptor binding, functional activity and selectivity in N-benzyltryptamines.
Toro-Sazo M, Brea J, Loza MI, Cimadevila M, Cassels BK. Toro-Sazo M, et al. PLoS One. 2019 Jan 10;14(1):e0209804. doi: 10.1371/journal.pone.0209804. eCollection 2019. PLoS One. 2019. PMID: 30629611 Free PMC article.
References
- Griffiths P, Evans-Brown M, Sedefov R, Getting up to speed with the public health and regulatory challenges posed by new psychoactive substances in the information age. Addiction 2013; 108:1700–1703. - PubMed
- EMCDDA. European Drug Report. European Monitoring Centre for Drugs and Drug Addiction, 2015. - PubMed
- DEA. Title 21 United States Code Controlled Substances Act, Part B, Section 812, Schedule 1(c) hallucinogenic substances, 2017.
- Lawn W, Barratt M, Williams M, Horne A, Winstock A, The NBOMe hallucinogenic drug series: Patterns of use, characteristics of users and self-reported effects in a large international sample. J Psychopharmacology 2014; 28:780–788. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources